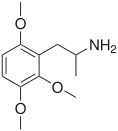
Trimethoxyamphetamine
TMAs, also known as trimethoxyamphetamines, are a family of isomeric psychedelic hallucinogenic drugs. There exist six different TMAs that differ only in the position of the three methoxy groups: TMA, TMA-2, TMA-3, TMA-4, TMA-5, and TMA-6. The TMAs are analogs of the phenethylamine cactus alkaloid mescaline. The TMAs are substituted amphetamines, however, their action does not resemble that of the unsubstituted compound amphetamine, which is a stimulant and not a psychedelic. It is reported that some TMA's elicit a range of emotions ranging from sadness to empathy and euphoria. TMA was first synthesized by Hey, in 1947.[1] Synthesis data as well as human activity data has been published in the book PiHKAL (Phenethylamines i Have Known And Loved).
The most important TMA compound from a pharmacological standpoint is TMA-2, as this isomer has been much more widely used as a recreational drug and sold on the grey market as a so-called "research chemical"; TMA (sometimes referred to as "mescalamphetamine" or TMA-1) and TMA-6 have also been used in this way to a lesser extent. These three isomers are significantly more active as hallucinogenic drugs, and have consequently been placed onto the illegal drug schedules in some countries such as the Netherlands and Japan. The other three isomers TMA-3, TMA-4 and TMA-5 are not known to have been used as recreational drugs to any great extent, and remain obscure scientific curiosities.
TMAs
|
|
| ||||||||||||||||||||||||||||||
|
|
| ||||||||||||||||||||||||||||||||
Note: As they are isomers the TMAs have the same totals formula, C12H19NO3, and the same molecular mass, 225.28 g/mol.
Properties
| Compound | Pattern | Dose | Duration |
|---|---|---|---|
| TMA | 3,4,5 | 100 – 250 mg | 6 - 8 h |
| TMA-2 | 2,4,5 | 20 – 40 mg | 8 - 12 h |
| TMA-3 | 2,3,4 | > 100 mg | unknown |
| TMA-4 | 2,3,5 | > 80 mg | ~ 6 h |
| TMA-5 | 2,3,6 | ≥ 30 mg | 8 - 10 h |
| TMA-6 | 2,4,6 | 25 – 50 mg | 12 - 16 h |
Legality
Sweden
Sveriges riksdag added TMA-2 to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of Dec 30, 1999, published by Medical Products Agency in their regulation LVFS 2004:3 listed as 2,4,5-trimetoxiamfetamin (TMA-2).[2]
See also
References
- Hey, P (1947). Quart. J. Pharm. Pharmacol. 20: 129. Missing or empty
|title=(help) - http://www.lakemedelsverket.se/upload/lvfs/LVFS_2004-3.pdf
External links
- PiHKAL entries:
- Erowid TMA vault
- EMCDDA Report on the risk assessment of TMA-2 in the framework of the joint action on new synthetic drugs
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| σ1 |
|
|---|---|
| σ2 |
|
| Unsorted |
|
See also: Receptor/signaling modulators | |
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|