Cobalamin biosynthesis
Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.
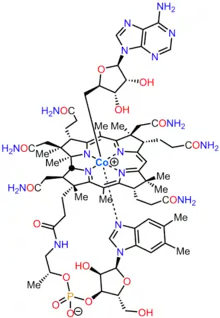

Cobalamin
Cobalamin (vitamin B12) is the largest and most structurally complex vitamin. It consists of a modified tetrapyrrole, a corrin, with a centrally chelated cobalt and is usually found in one of two biologically active forms: methylcobalamin and adenosylcobalamin. Most prokaryotes, as well as animals, have cobalamin-dependent enzymes that use it as a cofactor, whereas plants and fungi do not use it. In bacteria and archaea, these enzymes include methionine synthase, ribonucleotide reductase, glutamate and methylmalonyl-CoA mutases, ethanolamine ammonia-lyase, and diol dehydratase.[1] In certain mammals, cobalamin is obtained through the diet, and is required for methionine synthase and methylmalonyl-CoA mutase.[2] In humans, it plays essential roles in folate metabolism and in the synthesis of the citric acid cycle intermediate, succinyl-CoA.[3]
Overview of cobalamin biosynthesis
There are at least two distinct cobalamin biosynthetic pathways in bacteria:[4]
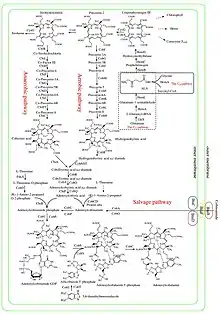 Biosynthetic pathways to Vitamin B12 from aminolevulinic acid (ALA) in bacteria and archaea |
 Vitamin B12 (as cyano cobalamin) and its parent cobyric acid |
- Aerobic pathway that requires oxygen and in which cobalt is inserted late in the pathway;[5][6] found in Pseudomonas denitrificans and Rhodobacter capsulatus.
- Anaerobic pathway in which cobalt insertion is the first committed step towards cobalamin synthesis;[7][8][9] found in Salmonella typhimurium, Bacillus megaterium, and Propionibacterium freudenreichii subsp. shermanii.
Either pathway can be divided into two parts:
- Corrin ring synthesis leading to cobyrinic acid, with seven carboxylate groups. In the anaerobic pathway this already contains cobalt but in the aerobic pathway the material formed at that stage is hydrogenobyrinic acid, without the bound cobalt.[10][11][12]
- Insertion of cobalt, where not already present; formation of amides on all but one of the carboxylate groups to give cobyric acid; attachment of an adenosyl group as ligand to the cobalt; attachment of an aminopropanol sidechain to the one free carboxylic group and assembly of the nucleotide loop which will provide the second ligand for the cobalt.[12][13]
A further type of synthesis occurs through a salvage pathway, where outside corrinoids are absorbed to make B12.[12] Species from the following genera and the following individual species are known to synthesize cobalamin: Propionibacterium shermanii, Pseudomonas denitrificans, Streptomyces griseus, Acetobacterium, Aerobacter, Agrobacterium, Alcaligenes, Azotobacter, Bacillus, Clostridium, Corynebacterium, Flavobacterium, Lactobacillus, Micromonospora, Mycobacterium, Nocardia, Proteus, Rhizobium, Salmonella, Serratia, Streptococcus and Xanthomonas.[14][15]
Detail of steps up to formation of uroporphyrinogen III
In the early steps of the biosynthesis, a tetrapyrrolic structural framework is created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethylbilane to uroporphyrinogen III. The latter is the first macrocyclic intermediate common to haem, chlorophyll, sirohaem and cobalamin itself.[6][16][17]
Detail of steps from uroporphyrinogen III to cob(II)yrinic acid a,c-diamide in aerobic organisms
The biosynthesis of cobalamin diverges from that of haem and chlorophyll at uroporphrinogen III: its transformation involves the sequential addition of methyl (CH3) groups to give intermediates that were given trivial names according to the number of these groups that have been incorporated. Hence, the first intermediate is precorrin-1, the next is precorrin-2 and so on. The incorporation of all eight additional methyl groups which occur in cobyric acid was investigated using 13C methyl-labelled S-adenosyl methionine. It was not until scientists at Rhône-Poulenc Rorer used a genetically-engineered strain of Pseudomonas denitrificans, in which eight of the cob genes involved in the biosynthesis of the vitamin had been overexpressed, that the complete sequence of methylation and other steps could be determined, thus fully establishing all the intermediates in the pathway.[18][19]
From uroporphyrinogen III to precorrin-2
The enzyme CobA catalyses the two chemical reactions EC 2.1.1.107[20]
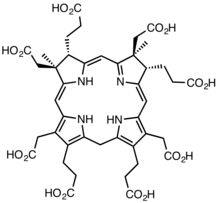
- (1a) uroporphyrinogen III + S-adenosyl methionine precorrin-1 + S-adenosyl-L-homocysteine
- (1b) precorrin-1 + S-adenosyl methionine precorrin-2 + S-adenosyl-L-homocysteine
From precorrin-2 to precorrin-3A
The enzyme CobI catalyzes the reaction EC 2.1.1.130[18]
- precorrin-2 + S-adenosyl methionine precorrin-3A + S-adenosyl-L-homocysteine
From precorrin-3A to precorrin-3B
The enzyme CobG catalyzes the reaction EC 1.14.13.83[18]
- precorrin-3A + NADH + H+ + O2 precorrin-3B + NAD+ + H2O
This enzyme is an oxidoreductase that requires oxygen and hence the reaction can only operate under aerobic conditions. The naming of these precorrins as 3A and 3B reflects the fact that each contains three more methyl groups than uroporphyrinogen III but with different structures: in particular, precorrin-3B has an internal γ-lactone ring formed from the ring A acetic acid sidechain closing back on to the macrocycle.
From precorrin-3B to precorrin-4
The enzyme CobJ continues the theme of methyl group insertion by catalysing the reaction EC 2.1.1.131[18]
- precorrin-3B + S-adenosyl methionine precorrin-4 + S-adenosyl-L-homocysteine

Importantly, during this step the macrocycle ring-contracts so that the product contains for the first time the corrin core which characterises cobalamin.
From precorrin-4 to precorrin-5
Methyl group insertions continue when the enzyme CobM catalyses the reaction EC 2.1.1.133[21]
- precorrin-4 + S-adenosyl methionine precorrin-5 + S-adenosyl-L-homocysteine
The newly-inserted methyl group is added to ring C at the carbon attached to the methylene (CH2) bridge to ring B. This is not its final location on cobalamin as a later step involves its rearrangement to an adjacent ring carbon.
From precorrin-5 to precorrin-6A
The enzyme CobF catalyzes the reaction EC 2.1.1.152[21]
- precorrin-5 + S-adenosyl methionine + H2O precorrin-6A + S-adenosyl-L-homocysteine + acetate
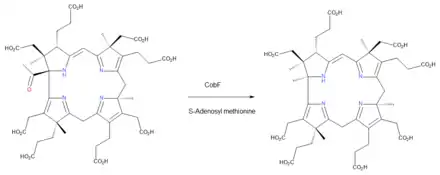
This conversion removes the acetyl group located at position 1 of the ring system in precorrin-4 and replaces it with a newly-introduced methyl group. The name of the product, precorrin-6A, reflects the fact that six methyl groups in total have been added to uroporphyrinogen III up to this point. However, since one of these has been extruded with the acetate group, the structure of precorrin-6A contains just the remaining five.
From precorrin-6A to precorrin-6B
The enzyme CobK now reduces a double bond in ring D by catalysing the reaction EC 1.3.1.54[21]
- precorrin-6A + NADPH + H+ precorrin-6B + NADP+
Precorrin-6B therefore differs in structure from precorrin-6A only by having an extra two hydrogen atoms.
From precorrin-6B to precorrin-8
The enzyme CobL has two active sites, one catalysing two methyl group additions and the other the decarboxylation of the CH2COOH group on ring D, so that this substituent becomes a simple methyl group EC 2.1.1.132[21]
- precorrin-6B + 2 S-adenosyl methionine precorrin-8X + 2 S-adenosyl-L-homocysteine + CO2
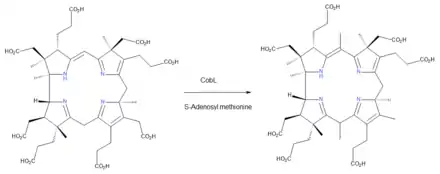
From precorrin-8 to hydrogenobyrinic acid
The enzyme CobH catalyzes a rearrangement reaction EC 5.4.99.61[22]
- precorrin-8X hydrogenobyrinate
The result is that the methyl group that had been added to ring C is isomerised to its final location, an example of intramolecular transfer.
From hydrogenobyrinic acid to hydrogenobyrinic acid a,c-diamide
The next enzyme in the pathway, CobB, converts two of the eight carboxylic acid groups into their primary amides in the reaction EC 6.3.5.9[23]
- hydrogenobyrinic acid + 2 ATP + 2 glutamine + 2 H2O hydrogenobyrinic acid a,c-diamide + 2 ADP + 2 phosphate + 2 glutamic acid
From hydrogenobyrinic acid a,c-diamide to cob(II)yrinic acid a,c-diamide
Cobalt(II) insertion into the macrocycle is catalysed by the enzyme Cobalt chelatase (CobNST) in the reaction EC 6.6.1.2[24]
- hydrogenobyrinic acid a,c-diamide + Co2+ + ATP + H2O cob(II)yrinic acid a,c-diamide + ADP + phosphate + H+
It is at this stage that the aerobic pathway and the anaerobic pathway merge, with later steps being chemically identical.
Detail of steps from uroporphyrinogen III to cob(II)yrinic acid a,c-diamide in anaerobic organisms
Many of the steps beyond uroporphyrinogen III in anaerobic organisms such as Bacillus megaterium involve chemically similar but genetically distinct transformations to those in the aerobic pathway.[9][25]
From precorrin-2 to cobalt-sirohydrochlorin
The key difference in the pathways is that cobalt is inserted early in anaerobic organisms by first oxidising precorrin-2 to its fully aromatised form sirohydrochlorin and then to that compound's cobalt(II) complex.[26] The reactions are catalysed by CysG EC 1.3.1.76 and Sirohydrochlorin cobaltochelatase EC 4.99.1.3.[27]
From cobalt-sirohydrochlorin to cobalt-factor III
As in the aerobic pathway, the third methyl group is introduced by a methyltransferase enzyme, CbiL in the reaction EC 2.1.1.151[26]
- cobalt-sirohydrochlorin + S-adenosyl methionine cobalt-factor III + S-adenosyl-L-homocysteine
From cobalt-factor III to cobalt-precorrin-4
Methylation and ring contraction to form the corrin macrocycle occurs next EC 2.1.1.272, catalysed by the enzyme Cobalt-factor III methyltransferase (CbiH)[28]
- cobalt-factor III + S-adenosyl methionine cobalt-precorrin-4 + S-adenosyl-L-homocysteine

In this pathway, the resulting material has contains a δ-lactone, a six-membered ring, rather than the γ-lactone (five-membered ring) of precorrin-3B.
From cobalt-precorrin-4 to cobalt-precorrin-5A
The introduction of the methyl group at C-11 in the next step is catalysed by Cobalt-precorrin-4 methyltransferase (CbiF) in the reaction EC 2.1.1.271[29]
- cobalt-precorrin-4 + S-adenosyl methionine cobalt-precorrin-5 + S-adenosyl-L-homocysteine
From cobalt-precorrin-5A to cobalt-precorrin-5B
The scene is now set for the extrusion of the two-carbon fragment corresponding to the acetate released in the formation of precorrin-6A in the aerobic pathway. In this case the fragment released is acetaldehyde and this is catalysed by CbiG in the reaction EC 3.7.1.12[29]
- cobalt-precorrin-5A + H2O cobalt-precorrin-5B + acetaldehyde + 2 H+
From cobalt-precorrin-5B to cob(II)yrinic acid a,c-diamide
The steps from cobalt-precorrin-5B to cob(II)yrinic acid a,c-diamide in the anaerobic pathway are essentially chemically identical to those in the aerobic sequence. The intermediates are called cobalt-precorrin-6A, cobalt-precorrin-6B, cobalt-precorrin-8 and cobyrinic acid and the enzymes / reactions involved are Cobalt-precorrin-5B (C1)-methyltransferase (CbiD / EC 2.1.1.195);[30] Cobalt-precorrin-6A reductase (CbiJ / EC 1.3.1.106);[31] Cobalt-precorrin-7 (C15)-methyltransferase (decarboxylating) (CbiET / EC 2.1.1.196), Cobalt-precorrin-8 methylmutase (CbiC / EC 5.4.99.60) and CbiA / EC 6.3.5.11. The final enzyme forms cob(II)yrinic acid a,c-diamide as the two pathways converge.[12]
Detail of steps from cob(II)yrinic acid a,c-diamide to adenosylcobalamin
Aerobic and anaerobic organisms share the same chemical pathway beyond cob(II)yrinic acid a,c-diamide and this is illustrated for the cob gene products.
From cob(II)yrinic acid a,c-diamide to adenosylcobyric acid
The cobalt(II) is reduced to cobalt(I) by the enzyme Cob(II)yrinic acid a,c-diamide reductase (CobR, reaction EC 1.16.8.1) and then the enzyme Cob(I)yrinic acid a,c-diamide adenosyltransferase (CobO) attaches an adenosyl ligand to the metal in reaction EC 2.5.1.17. Next, the enzyme CobQ (reaction EC 6.3.5.10) converts all the carboxylic acids, except the propionic acid on ring D, to their primary amides.[6][21]
From adenosylcobyric acid to adenosylcobinamide phosphate
In aerobic organisms, the enzyme CobCD (reaction EC 6.3.1.10) now attaches (R)-1-amino-2-propanol (derived from threonine) to the propionic acid, forming adenosylcobinamide and the enzyme CobU (reaction EC 2.7.1.156) phosphorylates the terminal hydroxy group to form adenosylcobinamide phosphate.[21] The same final product is formed in anaerobic organisms by direct reaction of adenosylcobyric acid with (R)-1-amino-2-propanol O-2-phosphate (derived from threonine-O-phosphate by the enzyme CobD in reaction EC 4.1.1.81) catalysed by the enzyme CbiB.[12]
From adenosylcobinamide phosphate to adenosylcobalamin
In a separate branch of the pathway, 5,6-dimethylbenzimidazole is biosynthesised from flavin mononucleotide by the enzyme 5,6-dimethylbenzimidazole synthase (reaction EC 1.13.11.79) and converted by CobT in reaction EC 2.4.2.21 to alpha-ribazole 5' phosphate. Then the enzyme CobU (reaction EC 2.7.7.62) activates adenosylcobinamide phosphate by formation of adenosylcobinamide-GDP and CobV (reaction EC 2.7.8.26) links the two substrates to form Adenosylcobalamin-5'-phosphate. In the final step to the coenzyme, CobC removes the 5' phosphate group in the reaction EC 3.1.3.73[32][33]
- Adenosylcobalamin-5'-phosphate + H2O adenosylcobalamin + phosphate
The complete biosynthetic route involves a long linear path that requires about 25 contributing enzyme steps.
Other pathways of cobalamin metabolism
Salvage pathways in prokaryotes
Many prokaryotic species cannot biosynthesize adenosylcobalamin, but can make it from cobalamin. These organisms are capable of cobalamin transport into the cell and its conversion to the required coenzyme form.[34] Even organisms such as Salmonella typhimurium that can make cobalamin also assimilate it from external sources when available.[12][35][36][37] Uptake into cells is facilitated by ABC transporters which absorb the cobalamin through the cell membrane.[38]
Cobalamin metabolism in humans
In humans, dietary sources of cobalamin are bound after ingestion as transcobalamins.[39] They are then converted to the coenzyme forms in which they are used. Methylmalonic aciduria and homocystinuria type C protein is the enzyme which catalyzes the decyanation of cyanocobalamin as well as the dealkylation of alkylcobalamins including methylcobalamin and adenosylcobalamin.[40][41][42]
References
- Rodionov, Dmitry A.; Vitreschak, Alexey G.; Mironov, Andrey A.; Gelfand, Mikhail S. (2003). "Comparative Genomics of the Vitamin B12 Metabolism and Regulation in Prokaryotes". Journal of Biological Chemistry. 278 (42): 41148–41159. doi:10.1074/jbc.M305837200. PMID 12869542.
- Banerjee, Ruma (2006). "B12 Trafficking in Mammals: A Case for Coenzyme Escort Service". ACS Chemical Biology. 1 (3): 149–159. doi:10.1021/cb6001174. PMID 17163662.
- "Vitamin B12". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 4 June 2015. Retrieved 20 April 2020.
- Roessner, Charles A.; Santander, Patricio J.; Scott, A.Ian (2001). "Multiple biosynthetic pathways for vitamin B12: Variations on a central theme". Cofactor Biosynthesis. Vitamins & Hormones. 61. pp. 267–297. doi:10.1016/s0083-6729(01)61009-4. ISBN 9780127098616. PMID 11153269.
- Heldt, D.; Lawrence, A.D.; Lindenmeyer, M.; Deery, E.; Heathcote, P.; Rigby, S.E.; Warren, M.J. (2005). "Aerobic synthesis of vitamin B12: Ring contraction and cobalt chelation". Biochemical Society Transactions. 33 (4): 815–819. doi:10.1042/BST0330815. PMID 16042605. S2CID 37362827.
- R. Caspi (2013-09-25). "Pathway: adenosylcobalamin biosynthesis II (aerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- Roessner CA, Huang KX, Warren MJ, Raux E, Scott AI (June 2002). "Isolation and characterization of 14 additional genes specifying the anaerobic biosynthesis of cobalamin (vitamin B12) in Propionibacterium freudenreichii (P. shermanii)". Microbiology. 148 (Pt 6): 1845–53. doi:10.1099/00221287-148-6-1845. PMID 12055304.
- Frank, S.; Brindley, A.A.; Deery, E.; Heathcote, P.; Lawrence, A.D.; Leech, H.K.; Pickersgill, R.W.; Warren, M.J. (2005). "Anaerobic synthesis of vitamin B12: Characterization of the early steps in the pathway". Biochemical Society Transactions. 33 (4): 811–814. doi:10.1042/BST0330811. PMID 16042604.
- R. Caspi (2013-09-25). "Pathway: adenosylcobalamin biosynthesis I (anaerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- Battersby, A. R. (1993). "How Nature builds the pigments of life" (PDF). Pure and Applied Chemistry. 65 (6): 1113–1122. doi:10.1351/pac199365061113. S2CID 83942303.
- Battersby, A. R. (2000). "Tetrapyrroles: the Pigments of Life. A Millennium review". Nat. Prod. Rep. 17 (6): 507–526. doi:10.1039/B002635M. PMID 11152419.
- Fang, H; Kang, J; Zhang, D (30 January 2017). "Microbial production of vitamin B12: a review and future perspectives". Microbial Cell Factories. 16 (1): 15. doi:10.1186/s12934-017-0631-y. PMC 5282855. PMID 28137297.
- Raux E, Schubert HL, Warren MJ (December 2000). "Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum". Cell. Mol. Life Sci. 57 (13–14): 1880–93. doi:10.1007/PL00000670. PMID 11215515. S2CID 583311.
- Perlman D (1959). "Microbial synthesis of cobamides". Advances in Applied Microbiology. 1: 87–122. doi:10.1016/S0065-2164(08)70476-3. ISBN 9780120026012. PMID 13854292.
- Martens JH, Barg H, Warren MJ, Jahn D (March 2002). "Microbial production of vitamin B12". Applied Microbiology and Biotechnology. 58 (3): 275–85. doi:10.1007/s00253-001-0902-7. PMID 11935176. S2CID 22232461.
- Battersby AR, Fookes CJ, Matcham GW, McDonald E (May 1980). "Biosynthesis of the pigments of life: formation of the macrocycle". Nature. 285 (5759): 17–21. Bibcode:1980Natur.285...17B. doi:10.1038/285017a0. PMID 6769048. S2CID 9070849.
- Frank S, Brindley AA, Deery E, Heathcote P, Lawrence AD, Leech HK, et al. (August 2005). "Anaerobic synthesis of vitamin B12: characterization of the early steps in the pathway". Biochemical Society Transactions. 33 (Pt 4): 811–4. doi:10.1042/BST0330811. PMID 16042604.
- Debussche, L.; Thibaut, D.; Cameron, B.; Crouzet, J.; Blanche, F. (1993). "Biosynthesis of the corrin macrocycle of coenzyme B12 in Pseudomonas denitrificans". Journal of Bacteriology. 175 (22): 7430–7440. doi:10.1128/jb.175.22.7430-7440.1993. PMC 206888. PMID 8226690.
- Battersby A (2005). "Chapter 11: Discovering the wonder of how Nature builds its molecules". In Archer MD, Haley CD (eds.). The 1702 chair of chemistry at Cambridge: transformation and change. Cambridge University Press. pp. xvi, 257–82. ISBN 0521828732.
- Warren, M. J.; Roessner, C. A.; Santander, P. J.; Scott, A. I. (1990). "The Escherichia coli cysG gene encodes S-adenosylmethionine-dependent uroporphyrinogen III methylase". Biochemical Journal. 265 (3): 725–729. doi:10.1042/bj2650725. PMC 1133693. PMID 2407234.
- Warren, Martin J.; Raux, Evelyne; Schubert, Heidi L.; Escalante-Semerena, Jorge C. (2002). "The biosynthesis of adenosylcobalamin (Vitamin B12)". Natural Product Reports. 19 (4): 390–412. doi:10.1039/b108967f. PMID 12195810.
- Thibaut, D.; Couder, M.; Famechon, A.; Debussche, L.; Cameron, B.; Crouzet, J.; Blanche, F. (1992). "The final step in the biosynthesis of hydrogenobyrinic acid is catalyzed by the cobH gene product with precorrin-8x as the substrate". Journal of Bacteriology. 174 (3): 1043–1049. doi:10.1128/jb.174.3.1043-1049.1992. PMC 206186. PMID 1732194.
- Debussche, L.; Thibaut, D.; Cameron, B.; Crouzet, J.; Blanche, F. (1990). "Purification and characterization of cobyrinic acid a,c-diamide synthase from Pseudomonas denitrificans". Journal of Bacteriology. 172 (11): 6239–6244. doi:10.1128/jb.172.11.6239-6244.1990. PMC 526805. PMID 2172209.
- Debussche, L.; Couder, M.; Thibaut, D.; Cameron, B.; Crouzet, J.; Blanche, F. (1992). "Assay, purification, and characterization of cobaltochelatase, a unique complex enzyme catalyzing cobalt insertion in hydrogenobyrinic acid a,c-diamide during coenzyme B12 biosynthesis in Pseudomonas denitrificans". Journal of Bacteriology. 174 (22): 7445–7451. doi:10.1128/JB.174.22.7445-7451.1992. PMC 207441. PMID 1429466.
- Roessner, Charles A.; Scott, A. Ian (2006). "Fine-Tuning Our Knowledge of the Anaerobic Route to Cobalamin (Vitamin B12)". Journal of Bacteriology. 188 (21): 7331–7334. doi:10.1128/JB.00918-06. PMC 1636268. PMID 16936030.
- Moore, Simon J.; Warren, Martin J. (2012). "The anaerobic biosynthesis of vitamin B12". Biochemical Society Transactions. 40 (3): 581–586. doi:10.1042/BST20120066. PMID 22616870.
- Yin, Jiang; Xu, Linda X.; Cherney, Maia M.; Raux-Deery, Evelyne; Bindley, Amanda A.; Savchenko, Alexei; Walker, John R.; Cuff, Marianne E.; Warren, Martin J.; James, Michael N. G. (2006). "Crystal Structure of the Vitamin B12 Biosynthetic Cobaltochelatase, CbiXS, from Archaeoglobus Fulgidus". Journal of Structural and Functional Genomics. 7 (1): 37–50. doi:10.1007/s10969-006-9008-x. PMID 16835730. S2CID 6613060.
- Moore, Simon J.; Biedendieck, Rebekka; Lawrence, Andrew D.; Deery, Evelyne; Howard, Mark J.; Rigby, Stephen E. J.; Warren, Martin J. (2013). "Characterization of the Enzyme CbiH60Involved in Anaerobic Ring Contraction of the Cobalamin (Vitamin B12) Biosynthetic Pathway". Journal of Biological Chemistry. 288 (1): 297–305. doi:10.1074/jbc.M112.422535. PMC 3537027. PMID 23155054.
- Kajiwara, Yasuhiro; Santander, Patricio J.; Roessner, Charles A.; Pérez, Lisa M.; Scott, A. Ian (2006). "Genetically Engineered Synthesis and Structural Characterization of Cobalt−Precorrin 5A and −5B, Two New Intermediates on the Anaerobic Pathway to Vitamin B12: Definition of the Roles of the CbiF and CbiG Enzymes". Journal of the American Chemical Society. 128 (30): 9971–9978. doi:10.1021/ja062940a. PMID 16866557.
- Roessner, Charles A.; Williams, Howard J.; Scott, A. Ian (2005). "Genetically Engineered Production of 1-Desmethylcobyrinic Acid, 1-Desmethylcobyrinic Acida,c-Diamide, and Cobyrinic Acida,c-Diamide in Escherichia coli Implies a Role for CbiD in C-1 Methylation in the Anaerobic Pathway to Cobalamin". Journal of Biological Chemistry. 280 (17): 16748–16753. doi:10.1074/jbc.M501805200. PMID 15741157.
- Kim, Wonduck; Major, Tiffany A.; Whitman, William B. (2005). "Role of the precorrin 6-X reductase gene in cobamide biosynthesis in Methanococcus maripaludis". Archaea. 1 (6): 375–384. doi:10.1155/2005/903614. PMC 2685584. PMID 16243778.
- R. Caspi (2007-04-23). "Pathway: adenosylcobalamin biosynthesis from adenosylcobinamide-GDP I". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- Zayas, Carmen L.; Escalante-Semerena, Jorge C. (2007). "Reassessment of the Late Steps of Coenzyme B12 Synthesis in Salmonella enterica: Evidence that Dephosphorylation of Adenosylcobalamin-5′-Phosphate by the CobC Phosphatase is the Last Step of the Pathway". Journal of Bacteriology. 189 (6): 2210–2218. doi:10.1128/jb.01665-06. PMC 1899380. PMID 17209023.
- R. Caspi (2013-09-25). "Pathway: adenosylcobalamin salvage from cobalamin". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- Escalante-Semerena, J. C.; Suh, S. J.; Roth, J. R. (1990). "CobA function is required for both de novo cobalamin biosynthesis and assimilation of exogenous corrinoids in Salmonella typhimurium". Journal of Bacteriology. 172 (1): 273–280. doi:10.1128/jb.172.1.273-280.1990. PMC 208428. PMID 2403541.
- Woodson, Jesse D.; Zayas, Carmen L.; Escalante-Semerena, Jorge C. (2003). "A New Pathway for Salvaging the CoenzymeB12 Precursor Cobinamide in Archaea Requires Cobinamide-Phosphate Synthase (CbiB) Enzyme Activity". Journal of Bacteriology. 185 (24): 7193–7201. doi:10.1128/jb.185.24.7193-7201.2003. PMC 296239. PMID 14645280.
- Woodson, J. D.; Escalante-Semerena, J. C. (2004). "CbiZ, an amidohydrolase enzyme required for salvaging the coenzyme B12 precursor cobinamide in archaea". Proceedings of the National Academy of Sciences. 101 (10): 3591–3596. Bibcode:2004PNAS..101.3591W. doi:10.1073/pnas.0305939101. PMC 373507. PMID 14990804.
- Woodson, Jesse D.; Reynolds, April A.; Escalante-Semerena, Jorge C. (2005). "ABC Transporter for Corrinoids in Halobacterium sp. Strain NRC-1". Journal of Bacteriology. 187 (17): 5901–5909. doi:10.1128/JB.187.17.5901-5909.2005. PMC 1196138. PMID 16109931.
- R. Caspi (2013-09-25). "Pathway: cobalamin salvage (eukaryotic)". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- Hannibal, Luciana; Kim, Jihoe; Brasch, Nicola E.; Wang, Sihe; Rosenblatt, David S.; Banerjee, Ruma; Jacobsen, Donald W. (2009). "Processing of alkylcobalamins in mammalian cells: A role for the MMACHC (CBLC) gene product". Molecular Genetics and Metabolism. 97 (4): 260–266. doi:10.1016/j.ymgme.2009.04.005. PMC 2709701. PMID 19447654.
- Banerjee, Ruma; Gherasim, Carmen; Padovani, Dominique (2009). "The tinker, tailor, soldier in intracellular B12 trafficking". Current Opinion in Chemical Biology. 13 (4): 484–491. doi:10.1016/j.cbpa.2009.07.007. PMC 5750051. PMID 19665918.
- Quadros, Edward V. (2010). "Advances in the understanding of cobalamin assimilation and metabolism". British Journal of Haematology. 148 (2): 195–204. doi:10.1111/j.1365-2141.2009.07937.x. PMC 2809139. PMID 19832808.
Further reading
- Layer, Gunhild; Jahn, Dieter; Deery, Evelyne; Lawrence, Andrew D.; Warren, Martin J. (2010). "Biosynthesis of Heme and Vitamin B12". Comprehensive Natural Products II. pp. 445–499. doi:10.1016/B978-008045382-8.00144-1. ISBN 9780080453828.
External links
- Prof Sir Alan Battersby: the biosynthesis of Vitamin B12 St. Catharine's College, Cambridge, video