Porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.[1]
 | |
| Names | |
|---|---|
| IUPAC name
3-[5-(Aminomethyl)-4-(carboxymethyl)-1H-pyrrol-3-yl]propanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.970 |
| EC Number |
|
| MeSH | Porphobilinogen |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H14N2O4 | |
| Molar mass | 226.229 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
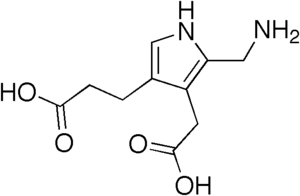
The structure of the molecule can be described as molecule of pyrrole with sidechains substituted for hydrogen atoms at positions 2, 3 and 4 in the ring (1 being the nitrogen atom); respectively, an aminomethyl group −CH
2−NH
2, an acetic acid (carboxymethyl) group −CH
2−COOH, and a propionic acid (carboxyethyl) group −CH
2−CH
2−COOH.
Biosynthesis
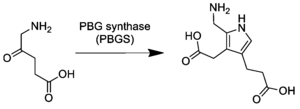
In the first step of the porphyrin biosynthesis pathway, porphobilinogen is generated from aminolevulinate (ALA) by the enzyme ALA dehydratase.
Metabolism
In the typical porphyrin biosynthesis pathway, four molecules of porphobilinogen are concatenated by carbons 2 and 5 of the pyrrole ring (adjacent to the nitrogen atom) into hydroxymethyl bilane by the enzyme porphobilinogen deaminase, also known as hydroxymethylbilane synthase.
Pathologies
Acute intermittent porphyria causes an increase in urinary porphobilinogen.[2]
References
- Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- Aarsand, AK; Petersen PH; Sandberg S (April 2006). "Estimation and application of biological variation of urinary delta-aminolevulinic acid and porphobilinogen in healthy individuals and in patients with acute intermittent porphyria". Clinical Chemistry. 52 (4): 650–656. doi:10.1373/clinchem.2005.060772. PMID 16595824.