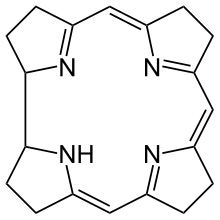
Corrin
Corrin is a heterocyclic compound. It is the parent macrocycle related to the substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 (cobalamins).[1]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H22N4 | |
| Molar mass | 306.40478 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Coordination chemistry
Upon deprotonation, the corrinoid ring is capable of binding cobalt. In vitamin B12, the resulting complex also features a benzimidazole-derived ligand, and the sixth site on the octahedron serves as the catalytic center.
The corrin ring resembles the porphyrin ring, which occurs in hemoglobin. Both feature four pyrrole-like subunits organized into a ring with a largely conjugated structure of alternating double and single bonds. In contrast to porphyrins, corrins lack one of the carbon groups that link the pyrrole-like units into a fully conjugated structure. With a conjugated system that extends only 3/4 of the way around the ring, and does not include any of the outer edge carbons, corrins have a number of non-conjugated sp3 carbons, making them more flexible than porphyrins and not as flat. A third closely related biological structure, the chlorin ring system found in chlorophyll, is intermediate between porphyrin and corrin, having 20 carbons like the porphyrins and a conjugated structure extending all the way around the central atom, but with only 6 of the 8 edge carbons participating.
Corroles (octadehydrocorrins) are fully aromatic derivatives of corrins.
References
- Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.