15-Cis-phytoene desaturase
15-cis-phytoene desaturases (PDS, plant-type phytoene desaturases) (EC 1.3.5.5, 15-cis-phytoene:plastoquinone oxidoreductase), are enzymes involved in the carotenoid biosynthesis in plants and cyanobacteria.[2] Phytoene desaturases are membrane-bound enzymes localized in plastids and introduce two double bonds into their colorless substrate phytoene by dehydrogenation and isomerize two additional double bonds.[3][4] This reaction starts a biochemical pathway involving three further enzymes (zeta-carotene isomerase, zeta-carotene desaturase and carotene cis-trans isomerase) called the poly-cis pathway and leads to the red colored lycopene. The homologous phytoene desaturase found in bacteria and fungi (CrtI) converts phytoene directly to lycopene by an all-trans pathway.[5]
| 15-cis-phytoene desaturase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 1.3.5.5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Biochemistry
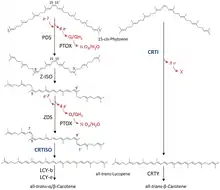
PDS converts 15-cis-phytoene into 9,15,9'-tri-cis-ζ-carotene through reduction of the enzymes non-covalently bound FAD cofactor.[6] This conversion introduces two additional double bonds at positions 11 and 11' of the carbon chain and isomerizes two adjacent already existing double bonds at positions 9 and 9' from trans to cis. The electrons involved in the reaction are subsequently transferred onto plastoquinone[7] and to plastid terminal oxidase PTOX ultimately coupling the desaturation to oxygen reduction. Disruption of this biosynthesis step results in albinism and stunted plant growth.[8]
Applications
Disruption of PDS function can be achieved by bleaching herbicides such as norflurazon[9] and fluridone.[10] These inhibitors occupy the binding pocket of plastoquinone within the enzyme thus blocking it from its function.[1] Due to the clear effect of PDS disruption in plants, the corresponding gene was targeted to showcase successful genome editing in fruit such as apples,[11] grapes[12] or bananas[13] using CRISPR/Cas9 systems. In rice, the natural PDS was supplemented by its bacterial homolog to create Golden Rice and thus increase the β-carotene content of the rice endosperm.
See also
References
- PDB: 5mog; Brausemann A, Gemmecker S, Koschmieder J, Ghisla S, Beyer P, Einsle O (August 2017). "Structure of Phytoene Desaturase Provides Insights into Herbicide Binding and Reaction Mechanisms Involved in Carotene Desaturation". Structure. 25 (8): 1222–1232.e3. doi:10.1016/j.str.2017.06.002. PMID 28669634.
- Fraser PD, Linden H, Sandmann G (May 1993). "Purification and reactivation of recombinant Synechococcus phytoene desaturase from an overexpressing strain of Escherichia coli". The Biochemical Journal. 291 ( Pt 3) (3): 687–92. doi:10.1042/bj2910687. PMC 1132422. PMID 8489496.
- Schneider C, Böger P, Sandmann G (July 1997). "Phytoene desaturase: heterologous expression in an active state, purification, and biochemical properties". Protein Expression and Purification. 10 (2): 175–9. doi:10.1006/prep.1997.0730. PMID 9226712.
- Breitenbach J, Sandmann G (March 2005). "zeta-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene". Planta. 220 (5): 785–93. doi:10.1007/s00425-004-1395-2. PMID 15503129.
- Moise AR, Al-Babili S, Wurtzel ET (31 October 2013). "Mechanistic aspects of carotenoid biosynthesis". Chemical Reviews. 114 (1): 164–193. doi:10.1021/cr400106y. PMC 3898671. PMID 24175570.
- Gemmecker S, Schaub P, Koschmieder J, Brausemann A, Drepper F, Rodriguez-Franco M, Ghisla S, Warscheid B, Einsle O, Beyer P (July 2015). "Phytoene Desaturase from Oryza sativa: Oligomeric Assembly, Membrane Association and Preliminary 3D-Analysis". PLOS ONE. 10 (7): e0131717. Bibcode:2015PLoSO..1031717G. doi:10.1371/journal.pone.0131717. PMC 4492965. PMID 26147209.
- Norris SR, Barrette TR, DellaPenna D (December 1995). "Genetic dissection of carotenoid synthesis in arabidopsis defines plastoquinone as an essential component of phytoene desaturation". The Plant Cell. 7 (12): 2139–49. doi:10.1105/tpc.7.12.2139. PMC 161068. PMID 8718624.
- Qin G, Gu H, Ma L, Peng Y, Deng XW, Chen Z, Qu LJ (May 2007). "Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis". Cell Research. 17 (5): 471–82. doi:10.1038/cr.2007.40. PMID 17486124.
- Breitenbach J, Zhu C, Sandmann G (November 2001). "Bleaching herbicide norflurazon inhibits phytoene desaturase by competition with the cofactors". Journal of Agricultural and Food Chemistry. 49 (11): 5270–2. doi:10.1021/jf0106751. PMID 11714315.
- Sandmann, Gerhard (2002). "2: Bleaching Herbicides: Action Mechanism in Carotenoid Biosynthesis, Structural Requirements and Engineering of Resistance". In Böger, Peter; Wakabayashi, Ko; Hirai, Kenji (eds.). Herbicide Classes in Development: Mode of Action, Targets, Genetic Engineering, Chemistry (1 ed.). Springer-Verlag Berlin Heidelberg. pp. 43–57. doi:10.1007/978-3-642-59416-8_2. ISBN 978-3-642-63972-2.
- Nishitani C, Hirai N, Komori S, Wada M, Okada K, Osakabe K, Yamamoto T, Osakabe Y (August 2016). "Efficient Genome Editing in Apple Using a CRISPR/Cas9 system". Scientific Reports. 6: 31481. Bibcode:2016NatSR...631481N. doi:10.1038/srep31481. PMC 4987624. PMID 27530958.
- Nakajima I, Ban Y, Azuma A, Onoue N, Moriguchi T, Yamamoto T, Toki S, Endo M (May 2017). "CRISPR/Cas9-mediated targeted mutagenesis in grape". PLOS ONE. 12 (5): e0177966. Bibcode:2017PLoSO..1277966N. doi:10.1371/journal.pone.0177966. PMC 5436839. PMID 28542349.
- Naim, Fatima; Dugdale, Benjamin; Kleidon, Jennifer; Brinin, Anthony; Shand, Kylie; Waterhouse, Peter; Dale, James (2018-07-09). "Gene editing the phytoene desaturase alleles of Cavendish banana using CRISPR/Cas9". Transgenic Research. 27 (5): 451–460. doi:10.1007/s11248-018-0083-0. ISSN 1573-9368. PMC 6156769. PMID 29987710.
External links
- 15-cis-phytoene+desaturase at the US National Library of Medicine Medical Subject Headings (MeSH)