Mammal
Mammals (from Latin mamma "breast") are a group of vertebrate animals constituting the class Mammalia (/məˈmeɪliə/), and characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur or hair, and three middle ear bones. These characteristics distinguish them from reptiles and birds, from which they diverged in the Carboniferous, over 300 million years ago. Around 6,400 extant species of mammals have been described. The largest orders are the rodents, bats and Eulipotyphla (hedgehogs, moles, shrews, and others). The next three are the Primates (including humans, apes, monkeys, and others), the Artiodactyla (cetaceans and even-toed ungulates), and the Carnivora (cats, dogs, seals, and others).
In terms of cladistics, which reflects evolutionary history, mammals are the only living members of the Synapsida; this clade, together with Sauropsida (reptiles and birds), constitutes the larger Amniota clade. The early synapsid mammalian ancestors were sphenacodont pelycosaurs, a group that included the non-mammalian Dimetrodon. At the end of the Carboniferous period around 300 million years ago, this group diverged from the sauropsid line that led to today's reptiles and birds. The line following the stem group Sphenacodontia split into several diverse groups of non-mammalian synapsids—sometimes incorrectly referred to as mammal-like reptiles—before giving rise to Therapsida in the Early Permian period. Mammals originated from cynodonts, an advanced group of therapsids, during the Late Triassic. The modern mammalian orders arose in the Paleogene and Neogene periods of the Cenozoic era, after the extinction of non-avian dinosaurs, and have been the dominant terrestrial animal group from 66 million years ago to the present.
The basic body type is quadruped, and most mammals use their four extremities for terrestrial locomotion; but in some, the extremities are adapted for life at sea, in the air, in trees, underground, or on two legs. Mammals range in size from the 30–40 mm (1.2–1.6 in) bumblebee bat to the 30 m (98 ft) blue whale—possibly the largest animal to have ever lived. Maximum lifespan varies from two years for the shrew to 211 years for the bowhead whale. All modern mammals give birth to live young, except the five species of monotremes, which are egg-laying mammals. The most species-rich group of mammals, the cohort called placentals, have a placenta, which enables the feeding of the fetus during gestation.
Most mammals are intelligent, with some possessing large brains, self-awareness, and tool use. Mammals can communicate and vocalize in several ways, including the production of ultrasound, scent-marking, alarm signals, singing, and echolocation. Mammals can organize themselves into fission-fusion societies, harems, and hierarchies—but can also be solitary and territorial. Most mammals are polygynous, but some can be monogamous or polyandrous.
Domestication of many types of mammals by humans played a major role in the Neolithic revolution, and resulted in farming replacing hunting and gathering as the primary source of food for humans. This led to a major restructuring of human societies from nomadic to sedentary, with more co-operation among larger and larger groups, and ultimately the development of the first civilizations. Domesticated mammals provided, and continue to provide, power for transport and agriculture, as well as food (meat and dairy products), fur, and leather. Mammals are also hunted and raced for sport, and are used as model organisms in science. Mammals have been depicted in art since Paleolithic times, and appear in literature, film, mythology, and religion. Decline in numbers and extinction of many mammals is primarily driven by human poaching and habitat destruction, primarily deforestation.
Classification

Mammal classification has been through several revisions since Carl Linnaeus initially defined the class, and at present, no classification system is universally accepted. McKenna & Bell (1997) and Wilson & Reader (2005) provide useful recent compendiums.[1] Simpson (1945)[2] provides systematics of mammal origins and relationships that had been taught universally until the end of the 20th century. However, since 1945, a large amount of new and more detailed information has gradually been found: The paleontological record has been recalibrated, and the intervening years have seen much debate and progress concerning the theoretical underpinnings of systematization itself, partly through the new concept of cladistics. Though field work and lab work progressively outdated Simpson's classification, it remains the closest thing to an official classification of mammals, despite its known issues.[3]
Most mammals, including the six most species-rich orders, belong to the placental group. The three largest orders in numbers of species are Rodentia: mice, rats, porcupines, beavers, capybaras, and other gnawing mammals; Chiroptera: bats; and Soricomorpha: shrews, moles, and solenodons. The next three biggest orders, depending on the biological classification scheme used, are the Primates: apes, monkeys, and lemurs; the Cetartiodactyla: whales and even-toed ungulates; and the Carnivora which includes cats, dogs, weasels, bears, seals, and allies.[4] According to Mammal Species of the World, 5,416 species were identified in 2006. These were grouped into 1,229 genera, 153 families and 29 orders.[4] In 2008, the International Union for Conservation of Nature (IUCN) completed a five-year Global Mammal Assessment for its IUCN Red List, which counted 5,488 species.[5] According to research published in the Journal of Mammalogy in 2018, the number of recognized mammal species is 6,495, including 96 recently extinct.[6]
Definitions
The word "mammal" is modern, from the scientific name Mammalia coined by Carl Linnaeus in 1758, derived from the Latin mamma ("teat, pap"). In an influential 1988 paper, Timothy Rowe defined Mammalia phylogenetically as the crown group of mammals, the clade consisting of the most recent common ancestor of living monotremes (echidnas and platypuses) and Therian mammals (marsupials and placentals) and all descendants of that ancestor.[7] Since this ancestor lived in the Jurassic period, Rowe's definition excludes all animals from the earlier Triassic, despite the fact that Triassic fossils in the Haramiyida have been referred to the Mammalia since the mid-19th century.[8] If Mammalia is considered as the crown group, its origin can be roughly dated as the first known appearance of animals more closely related to some extant mammals than to others. Ambondro is more closely related to monotremes than to therian mammals while Amphilestes and Amphitherium are more closely related to the therians; as fossils of all three genera are dated about 167 million years ago in the Middle Jurassic, this is a reasonable estimate for the appearance of the crown group.[9]
T.S. Kemp has provided a more traditional definition: "Synapsids that possess a dentary–squamosal jaw articulation and occlusion between upper and lower molars with a transverse component to the movement" or, equivalently in Kemp's view, the clade originating with the last common ancestor of Sinoconodon and living mammals.[10] The earliest known synapsid satisfying Kemp's definitions is Tikitherium, dated 225 Ma, so the appearance of mammals in this broader sense can be given this Late Triassic date.[11][12]
McKenna/Bell classification
In 1997, the mammals were comprehensively revised by Malcolm C. McKenna and Susan K. Bell, which has resulted in the McKenna/Bell classification. The authors worked together as paleontologists at the American Museum of Natural History. McKenna inherited the project from Simpson and, with Bell, constructed a completely updated hierarchical system, covering living and extinct taxa, that reflects the historical genealogy of Mammalia.[3] Their 1997 book, Classification of Mammals above the Species Level,[13] is a comprehensive work on the systematics, relationships and occurrences of all mammal taxa, living and extinct, down through the rank of genus, though molecular genetic data challenge several of the higher level groupings.
In the following list, extinct groups are labelled with a dagger (†).
Class Mammalia
- Subclass Prototheria: monotremes: echidnas and the platypus
- Subclass Theriiformes: live-bearing mammals and their prehistoric relatives
- Infraclass †Allotheria: multituberculates
- Infraclass †Eutriconodonta: eutriconodonts
- Infraclass Holotheria: modern live-bearing mammals and their prehistoric relatives
- Superlegion †Kuehneotheria
- Supercohort Theria: live-bearing mammals
- Cohort Marsupialia: marsupials
- Magnorder Australidelphia: Australian marsupials and the monito del monte
- Magnorder Ameridelphia: New World marsupials. Now considered paraphyletic, with shrew opossums being closer to australidelphians.[14]
- Cohort Placentalia: placentals
- Magnorder Xenarthra: xenarthrans
- Magnorder Epitheria: epitheres
- Superorder †Leptictida
- Superorder Preptotheria
- Grandorder Anagalida: lagomorphs, rodents and elephant shrews
- Grandorder Ferae: carnivorans, pangolins, †creodonts and relatives
- Grandorder Lipotyphla: insectivorans
- Grandorder Archonta: bats, primates, colugos and treeshrews
- Grandorder Ungulata: ungulates
- Order Tubulidentata incertae sedis: aardvark
- Mirorder Eparctocyona: †condylarths, whales and artiodactyls (even-toed ungulates)
- Mirorder †Meridiungulata: South American ungulates
- Mirorder Altungulata: perissodactyls (odd-toed ungulates), elephants, manatees and hyraxes
- Cohort Marsupialia: marsupials
Molecular classification of placentals
As of the early 21st century, molecular studies based on DNA analysis have suggested new relationships among mammal families. Most of these findings have been independently validated by retrotransposon presence/absence data.[15] Classification systems based on molecular studies reveal three major groups or lineages of placental mammals—Afrotheria, Xenarthra and Boreoeutheria—which diverged in the Cretaceous. The relationships between these three lineages is contentious, and all three possible hypotheses have been proposed with respect to which group is basal. These hypotheses are Atlantogenata (basal Boreoeutheria), Epitheria (basal Xenarthra) and Exafroplacentalia (basal Afrotheria).[16] Boreoeutheria in turn contains two major lineages—Euarchontoglires and Laurasiatheria.
Estimates for the divergence times between these three placental groups range from 105 to 120 million years ago, depending on the type of DNA used (such as nuclear or mitochondrial)[17] and varying interpretations of paleogeographic data.[16]
| Mammalia |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The cladogram above is based on Tarver et al. (2016)[18]
Group I: Superorder Afrotheria[19]
- Clade Afroinsectiphilia
- Order Macroscelidea: elephant shrews (Africa)
- Order Afrosoricida: tenrecs and golden moles (Africa)
- Order Tubulidentata: aardvark (Africa south of the Sahara)
- Clade Paenungulata
- Order Hyracoidea: hyraxes or dassies (Africa, Arabia)
- Order Proboscidea: elephants (Africa, Southeast Asia)
- Order Sirenia: dugong and manatees (cosmopolitan tropical)
Group II: Superorder Xenarthra[19]
- Order Pilosa: sloths and anteaters (neotropical)
- Order Cingulata: armadillos and extinct relatives (Americas)
Group III: Magnaorder Boreoeutheria[19]
- Superorder: Euarchontoglires (Supraprimates)
- Grandorder Euarchonta
- Order Scandentia: treeshrews (Southeast Asia).
- Order Dermoptera: flying lemurs or colugos (Southeast Asia)
- Order Primates: lemurs, bushbabies, monkeys, apes, humans (cosmopolitan)
- Grandorder Glires
- Order Lagomorpha: pikas, rabbits, hares (Eurasia, Africa, Americas)
- Order Rodentia: rodents (cosmopolitan)
- Grandorder Euarchonta
- Superorder: Laurasiatheria
- Order Eulipotyphla: shrews, hedgehogs, moles, solenodons
- Clade Scrotifera
- Order Chiroptera: bats (cosmopolitan)
- Clade Fereuungulata
- Clade Ferae
- Clade Euungulata
- Order Cetartiodactyla: cetaceans (whales, dolphins and porpoises) and even-toed ungulates, including pigs, cattle, deer and giraffes
- Order Perissodactyla: odd-toed ungulates, including horses, donkeys, zebras, tapirs and rhinoceroses
Evolution
Origins
Synapsida, a clade that contains mammals and their extinct relatives, originated during the Pennsylvanian subperiod (~323 million to ~300 million years ago), when they split from reptilian and avian lineages. Crown group mammals evolved from earlier mammaliaforms during the Early Jurassic. The cladogram takes Mammalia to be the crown group.[20]
| Mammaliaformes |
| ||||||||||||||||||||||||||||||||||||||||||||||||
Evolution from amniotes

The first fully terrestrial vertebrates were amniotes. Like their amphibious tetrapod predecessors, they had lungs and limbs. Amniotic eggs, however, have internal membranes that allow the developing embryo to breathe but keep water in. Hence, amniotes can lay eggs on dry land, while amphibians generally need to lay their eggs in water.
The first amniotes apparently arose in the Pennsylvanian subperiod of the Carboniferous. They descended from earlier reptiliomorph amphibious tetrapods,[21] which lived on land that was already inhabited by insects and other invertebrates as well as ferns, mosses and other plants. Within a few million years, two important amniote lineages became distinct: the synapsids, which would later include the common ancestor of the mammals; and the sauropsids, which now include turtles, lizards, snakes, crocodilians and dinosaurs (including birds).[22] Synapsids have a single hole (temporal fenestra) low on each side of the skull. One synapsid group, the pelycosaurs, included the largest and fiercest animals of the early Permian.[23] Nonmammalian synapsids are sometimes (inaccurately) called "mammal-like reptiles".[24][25]
Therapsids, a group of synapsids, descended from pelycosaurs in the Middle Permian, about 265 million years ago, and became the dominant land vertebrates.[24] They differ from basal eupelycosaurs in several features of the skull and jaws, including: larger skulls and incisors which are equal in size in therapsids, but not for eupelycosaurs.[24] The therapsid lineage leading to mammals went through a series of stages, beginning with animals that were very similar to their pelycosaur ancestors and ending with probainognathian cynodonts, some of which could easily be mistaken for mammals. Those stages were characterized by:[26]
- The gradual development of a bony secondary palate.
- Progression towards an erect limb posture, which would increase the animals' stamina by avoiding Carrier's constraint. But this process was slow and erratic: for example, all herbivorous nonmammaliaform therapsids retained sprawling limbs (some late forms may have had semierect hind limbs); Permian carnivorous therapsids had sprawling forelimbs, and some late Permian ones also had semisprawling hindlimbs. In fact, modern monotremes still have semisprawling limbs.
- The dentary gradually became the main bone of the lower jaw which, by the Triassic, progressed towards the fully mammalian jaw (the lower consisting only of the dentary) and middle ear (which is constructed by the bones that were previously used to construct the jaws of reptiles).
First mammals
The Permian–Triassic extinction event about 252 million years ago, which was a prolonged event due to the accumulation of several extinction pulses, ended the dominance of carnivorous therapsids.[27] In the early Triassic, most medium to large land carnivore niches were taken over by archosaurs[28] which, over an extended period (35 million years), came to include the crocodylomorphs,[29] the pterosaurs and the dinosaurs;[30] however, large cynodonts like Trucidocynodon and traversodontids still occupied large sized carnivorous and herbivorous niches respectively. By the Jurassic, the dinosaurs had come to dominate the large terrestrial herbivore niches as well.[31]
The first mammals (in Kemp's sense) appeared in the Late Triassic epoch (about 225 million years ago), 40 million years after the first therapsids. They expanded out of their nocturnal insectivore niche from the mid-Jurassic onwards;[32] The Jurassic Castorocauda, for example, was a close relative of true mammals that had adaptations for swimming, digging and catching fish.[33] Most, if not all, are thought to have remained nocturnal (the nocturnal bottleneck), accounting for much of the typical mammalian traits.[34] The majority of the mammal species that existed in the Mesozoic Era were multituberculates, eutriconodonts and spalacotheriids.[35] The earliest known metatherian is Sinodelphys, found in 125 million-year-old Early Cretaceous shale in China's northeastern Liaoning Province. The fossil is nearly complete and includes tufts of fur and imprints of soft tissues.[36]

The oldest known fossil among the Eutheria ("true beasts") is the small shrewlike Juramaia sinensis, or "Jurassic mother from China", dated to 160 million years ago in the late Jurassic.[37] A later eutherian relative, Eomaia, dated to 125 million years ago in the early Cretaceous, possessed some features in common with the marsupials but not with the placentals, evidence that these features were present in the last common ancestor of the two groups but were later lost in the placental lineage.[38] In particular, the epipubic bones extend forwards from the pelvis. These are not found in any modern placental, but they are found in marsupials, monotremes, other nontherian mammals and Ukhaatherium, an early Cretaceous animal in the eutherian order Asioryctitheria. This also applies to the multituberculates.[39] They are apparently an ancestral feature, which subsequently disappeared in the placental lineage. These epipubic bones seem to function by stiffening the muscles during locomotion, reducing the amount of space being presented, which placentals require to contain their fetus during gestation periods. A narrow pelvic outlet indicates that the young were very small at birth and therefore pregnancy was short, as in modern marsupials. This suggests that the placenta was a later development.[40]
One of the earliest known monotremes was Teinolophos, which lived about 120 million years ago in Australia.[41] Monotremes have some features which may be inherited from the original amniotes such as the same orifice to urinate, defecate and reproduce (cloaca)—as lizards and birds also do—[42] and they lay eggs which are leathery and uncalcified.[43]
Earliest appearances of features
Hadrocodium, whose fossils date from approximately 195 million years ago, in the early Jurassic, provides the first clear evidence of a jaw joint formed solely by the squamosal and dentary bones; there is no space in the jaw for the articular, a bone involved in the jaws of all early synapsids.[44]

The earliest clear evidence of hair or fur is in fossils of Castorocauda and Megaconus, from 164 million years ago in the mid-Jurassic. In the 1950s, it was suggested that the foramina (passages) in the maxillae and premaxillae (bones in the front of the upper jaw) of cynodonts were channels which supplied blood vessels and nerves to vibrissae (whiskers) and so were evidence of hair or fur;[45][46] it was soon pointed out, however, that foramina do not necessarily show that an animal had vibrissae, as the modern lizard Tupinambis has foramina that are almost identical to those found in the nonmammalian cynodont Thrinaxodon.[25][47] Popular sources, nevertheless, continue to attribute whiskers to Thrinaxodon.[48] Studies on Permian coprolites suggest that non-mammalian synapsids of the epoch already had fur, setting the evolution of hairs possibly as far back as dicynodonts.[49]
When endothermy first appeared in the evolution of mammals is uncertain, though it is generally agreed to have first evolved in non-mammalian therapsids.[49][50] Modern monotremes have lower body temperatures and more variable metabolic rates than marsupials and placentals,[51] but there is evidence that some of their ancestors, perhaps including ancestors of the therians, may have had body temperatures like those of modern therians.[52] Likewise, some modern therians like afrotheres and xenarthrans have secondarily developed lower body temperatures.[53]
The evolution of erect limbs in mammals is incomplete—living and fossil monotremes have sprawling limbs. The parasagittal (nonsprawling) limb posture appeared sometime in the late Jurassic or early Cretaceous; it is found in the eutherian Eomaia and the metatherian Sinodelphys, both dated to 125 million years ago.[54] Epipubic bones, a feature that strongly influenced the reproduction of most mammal clades, are first found in Tritylodontidae, suggesting that it is a synapomorphy between them and mammaliformes. They are omnipresent in non-placental mammaliformes, though Megazostrodon and Erythrotherium appear to have lacked them.[55]
It has been suggested that the original function of lactation (milk production) was to keep eggs moist. Much of the argument is based on monotremes, the egg-laying mammals.[56][57] In human females, mammary glands become fully developed during puberty, regardless of pregnancy.[58]
Rise of the mammals
Therian mammals took over the medium- to large-sized ecological niches in the Cenozoic, after the Cretaceous–Paleogene extinction event approximately 66 million years ago emptied ecological space once filled by non-avian dinosaurs and other groups of reptiles, as well as various other mammal groups,[59] and underwent an exponential increase in body size (megafauna).[60] Then mammals diversified very quickly; both birds and mammals show an exponential rise in diversity.[59] For example, the earliest known bat dates from about 50 million years ago, only 16 million years after the extinction of the non-avian dinosaurs.[61]
Molecular phylogenetic studies initially suggested that most placental orders diverged about 100 to 85 million years ago and that modern families appeared in the period from the late Eocene through the Miocene.[62] However, no placental fossils have been found from before the end of the Cretaceous.[63] The earliest undisputed fossils of placentals comes from the early Paleocene, after the extinction of the non-avian dinosaurs.[63] In particular, scientists have identified an early Paleocene animal named Protungulatum donnae as one of the first placental mammals.[64] however it has been reclassified as a non-placental eutherian.[65] Recalibrations of genetic and morphological diversity rates have suggested a Late Cretaceous origin for placentals, and a Paleocene origin for most modern clades.[66]
The earliest known ancestor of primates is Archicebus achilles[67] from around 55 million years ago.[67] This tiny primate weighed 20–30 grams (0.7–1.1 ounce) and could fit within a human palm.[67]
Anatomy
Distinguishing features
Living mammal species can be identified by the presence of sweat glands, including those that are specialized to produce milk to nourish their young.[68] In classifying fossils, however, other features must be used, since soft tissue glands and many other features are not visible in fossils.[69]
Many traits shared by all living mammals appeared among the earliest members of the group:
- Jaw joint – The dentary (the lower jaw bone, which carries the teeth) and the squamosal (a small cranial bone) meet to form the joint. In most gnathostomes, including early therapsids, the joint consists of the articular (a small bone at the back of the lower jaw) and quadrate (a small bone at the back of the upper jaw).[44]
- Middle ear – In crown-group mammals, sound is carried from the eardrum by a chain of three bones, the malleus, the incus and the stapes. Ancestrally, the malleus and the incus are derived from the articular and the quadrate bones that constituted the jaw joint of early therapsids.[70]
- Tooth replacement – Teeth can be replaced once (diphyodonty) or (as in toothed whales and murid rodents) not at all (monophyodonty).[71] Elephants, manatees, and kangaroos continually grow new teeth throughout their life (polyphyodonty).[72]
- Prismatic enamel – The enamel coating on the surface of a tooth consists of prisms, solid, rod-like structures extending from the dentin to the tooth's surface.[73]
- Occipital condyles – Two knobs at the base of the skull fit into the topmost neck vertebra; most other tetrapods, in contrast, have only one such knob.[74]
For the most part, these characteristics were not present in the Triassic ancestors of the mammals.[75] Nearly all mammaliaforms possess an epipubic bone, the exception being modern placentals.[76]
Sexual dimorphism
On average, male mammals are larger than females, with males being at least 10% larger than females in over 45% of investigated species. Most mammalian orders are also exhibit male-biased sexual dimorphism, although some orders do not show any bias or are significantly female-biased (Lagomorpha). Sexual size dimorphism increases with body size across mammals (Rensch's rule), suggesting that there are parallel selection pressures on both male and female size. Male-biased dimorphism relates to sexual selection on males through male–male competition for females, as there is a positive correlation between the degree of sexual selection, as indicated by mating systems, and the degree of male-biased size dimorphism. The degree of sexual selection is also positively correlated with male and female size across mammals. Further, a parallel selection pressure on female mass is identified in that age at weaning is significantly higher in more polygynous species, even when correcting for body mass. Also, reproductive rate is lower for larger females, indicating that fecundity selection selects for smaller females in mammals. Although these patterns hold across mammals as a whole, there is considerable variation across orders.[77]
Biological systems

The majority of mammals have seven cervical vertebrae (bones in the neck). The exceptions are the manatee and the two-toed sloth, which have six, and the three-toed sloth which has nine.[78] All mammalian brains possess a neocortex, a brain region unique to mammals.[79] Placental brains have a corpus callosum, unlike monotremes and marsupials.[80]
The lungs of mammals are spongy and honeycombed. Breathing is mainly achieved with the diaphragm, which divides the thorax from the abdominal cavity, forming a dome convex to the thorax. Contraction of the diaphragm flattens the dome, increasing the volume of the lung cavity. Air enters through the oral and nasal cavities, and travels through the larynx, trachea and bronchi, and expands the alveoli. Relaxing the diaphragm has the opposite effect, decreasing the volume of the lung cavity, causing air to be pushed out of the lungs. During exercise, the abdominal wall contracts, increasing pressure on the diaphragm, which forces air out quicker and more forcefully. The rib cage is able to expand and contract the chest cavity through the action of other respiratory muscles. Consequently, air is sucked into or expelled out of the lungs, always moving down its pressure gradient.[81][82] This type of lung is known as a bellows lung due to its resemblance to blacksmith bellows.[82]
The mammalian heart has four chambers, two upper atria, the receiving chambers, and two lower ventricles, the discharging chambers.[83] The heart has four valves, which separate its chambers and ensures blood flows in the correct direction through the heart (preventing backflow). After gas exchange in the pulmonary capillaries (blood vessels in the lungs), oxygen-rich blood returns to the left atrium via one of the four pulmonary veins. Blood flows nearly continuously back into the atrium, which acts as the receiving chamber, and from here through an opening into the left ventricle. Most blood flows passively into the heart while both the atria and ventricles are relaxed, but toward the end of the ventricular relaxation period, the left atrium will contract, pumping blood into the ventricle. The heart also requires nutrients and oxygen found in blood like other muscles, and is supplied via coronary arteries.[84]
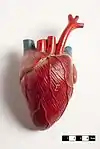

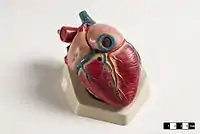
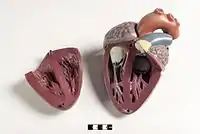

The integumentary system (skin) is made up of three layers: the outermost epidermis, the dermis and the hypodermis. The epidermis is typically 10 to 30 cells thick; its main function is to provide a waterproof layer. Its outermost cells are constantly lost; its bottommost cells are constantly dividing and pushing upward. The middle layer, the dermis, is 15 to 40 times thicker than the epidermis. The dermis is made up of many components, such as bony structures and blood vessels. The hypodermis is made up of adipose tissue, which stores lipids and provides cushioning and insulation. The thickness of this layer varies widely from species to species;[85]:97 marine mammals require a thick hypodermis (blubber) for insulation, and right whales have the thickest blubber at 20 inches (51 cm).[86] Although other animals have features such as whiskers, feathers, setae, or cilia that superficially resemble it, no animals other than mammals have hair. It is a definitive characteristic of the class, though some mammals have very little.[85]:61
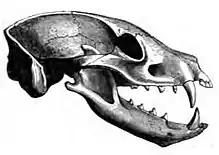

Herbivores have developed a diverse range of physical structures to facilitate the consumption of plant material. To break up intact plant tissues, mammals have developed teeth structures that reflect their feeding preferences. For instance, frugivores (animals that feed primarily on fruit) and herbivores that feed on soft foliage have low-crowned teeth specialized for grinding foliage and seeds. Grazing animals that tend to eat hard, silica-rich grasses, have high-crowned teeth, which are capable of grinding tough plant tissues and do not wear down as quickly as low-crowned teeth.[87] Most carnivorous mammals have carnassialiforme teeth (of varying length depending on diet), long canines and similar tooth replacement patterns.[88]
The stomach of even-toed ungulates (Artiodactyla) is divided into four sections: the rumen, the reticulum, the omasum and the abomasum (only ruminants have a rumen). After the plant material is consumed, it is mixed with saliva in the rumen and reticulum and separates into solid and liquid material. The solids lump together to form a bolus (or cud), and is regurgitated. When the bolus enters the mouth, the fluid is squeezed out with the tongue and swallowed again. Ingested food passes to the rumen and reticulum where cellulolytic microbes (bacteria, protozoa and fungi) produce cellulase, which is needed to break down the cellulose in plants.[89] Perissodactyls, in contrast to the ruminants, store digested food that has left the stomach in an enlarged cecum, where it is fermented by bacteria.[90] Carnivora have a simple stomach adapted to digest primarily meat, as compared to the elaborate digestive systems of herbivorous animals, which are necessary to break down tough, complex plant fibers. The caecum is either absent or short and simple, and the large intestine is not sacculated or much wider than the small intestine.[91]

The mammalian excretory system involves many components. Like most other land animals, mammals are ureotelic, and convert ammonia into urea, which is done by the liver as part of the urea cycle.[92] Bilirubin, a waste product derived from blood cells, is passed through bile and urine with the help of enzymes excreted by the liver.[93] The passing of bilirubin via bile through the intestinal tract gives mammalian feces a distinctive brown coloration.[94] Distinctive features of the mammalian kidney include the presence of the renal pelvis and renal pyramids, and of a clearly distinguishable cortex and medulla, which is due to the presence of elongated loops of Henle. Only the mammalian kidney has a bean shape, although there are some exceptions, such as the multilobed reniculate kidneys of pinnipeds, cetaceans and bears.[95][96] Most adult placental mammals have no remaining trace of the cloaca. In the embryo, the embryonic cloaca divides into a posterior region that becomes part of the anus, and an anterior region that has different fates depending on the sex of the individual: in females, it develops into the vestibule that receives the urethra and vagina, while in males it forms the entirety of the penile urethra.[96] However, the tenrecs, golden moles, and some shrews retain a cloaca as adults.[97] In marsupials, the genital tract is separate from the anus, but a trace of the original cloaca does remain externally.[96] Monotremes, which translates from Greek into "single hole", have a true cloaca.[98]
Sound production

As in all other tetrapods, mammals have a larynx that can quickly open and close to produce sounds, and a supralaryngeal vocal tract which filters this sound. The lungs and surrounding musculature provide the air stream and pressure required to phonate. The larynx controls the pitch and volume of sound, but the strength the lungs exert to exhale also contributes to volume. More primitive mammals, such as the echidna, can only hiss, as sound is achieved solely through exhaling through a partially closed larynx. Other mammals phonate using vocal folds, as opposed to the vocal cords seen in birds and reptiles. The movement or tenseness of the vocal folds can result in many sounds such as purring and screaming. Mammals can change the position of the larynx, allowing them to breathe through the nose while swallowing through the mouth, and to form both oral and nasal sounds; nasal sounds, such as a dog whine, are generally soft sounds, and oral sounds, such as a dog bark, are generally loud.[99]
Some mammals have a large larynx and thus a low-pitched voice, namely the hammer-headed bat (Hypsignathus monstrosus) where the larynx can take up the entirety of the thoracic cavity while pushing the lungs, heart, and trachea into the abdomen.[100] Large vocal pads can also lower the pitch, as in the low-pitched roars of big cats.[101] The production of infrasound is possible in some mammals such as the African elephant (Loxodonta spp.) and baleen whales.[102][103] Small mammals with small larynxes have the ability to produce ultrasound, which can be detected by modifications to the middle ear and cochlea. Ultrasound is inaudible to birds and reptiles, which might have been important during the Mesozoic, when birds and reptiles were the dominant predators. This private channel is used by some rodents in, for example, mother-to-pup communication, and by bats when echolocating. Toothed whales also use echolocation, but, as opposed to the vocal membrane that extends upward from the vocal folds, they have a melon to manipulate sounds. Some mammals, namely the primates, have air sacs attached to the larynx, which may function to lower the resonances or increase the volume of sound.[99]
The vocal production system is controlled by the cranial nerve nuclei in the brain, and supplied by the recurrent laryngeal nerve and the superior laryngeal nerve, branches of the vagus nerve. The vocal tract is supplied by the hypoglossal nerve and facial nerves. Electrical stimulation of the periaqueductal gray (PEG) region of the mammalian midbrain elicit vocalizations. The ability to learn new vocalizations is only exemplified in humans, seals, cetaceans, elephants and possibly bats; in humans, this is the result of a direct connection between the motor cortex, which controls movement, and the motor neurons in the spinal cord.[99]
Fur

The primary function of the fur of mammals is thermoregulation. Others include protection, sensory purposes, waterproofing, and camouflage.[104] Different types of fur serve different purposes:[85]:99
- Definitive – which may be shed after reaching a certain length
- Vibrissae – sensory hairs, most commonly whiskers
- Pelage – guard hairs, under-fur, and awn hair
- Spines – stiff guard hair used for defense (such as in porcupines)
- Bristles – long hairs usually used in visual signals. (such as a lion's mane)
- Velli – often called "down fur" which insulates newborn mammals
- Wool – long, soft and often curly
Thermoregulation
Hair length is not a factor in thermoregulation: for example, some tropical mammals such as sloths have the same length of fur length as some arctic mammals but with less insulation; and, conversely, other tropical mammals with short hair have the same insulating value as arctic mammals. The denseness of fur can increase an animal's insulation value, and arctic mammals especially have dense fur; for example, the musk ox has guard hairs measuring 30 cm (12 in) as well as a dense underfur, which forms an airtight coat, allowing them to survive in temperatures of −40 °C (−40 °F).[85]:162–163 Some desert mammals, such as camels, use dense fur to prevent solar heat from reaching their skin, allowing the animal to stay cool; a camel's fur may reach 70 °C (158 °F) in the summer, but the skin stays at 40 °C (104 °F).[85]:188 Aquatic mammals, conversely, trap air in their fur to conserve heat by keeping the skin dry.[85]:162–163

Coloration
Mammalian coats are colored for a variety of reasons, the major selective pressures including camouflage, sexual selection, communication, and thermoregulation. Coloration in both the hair and skin of mammals is mainly determined by the type and amount of melanin; eumelanins for brown and black colors and pheomelanin for a range of yellowish to reddish colors, giving mammals an earth tone.[105][106] Some mammals, like the mandrill, have more vibrant colors due to structural coloration.[107] Many sloths appear green because their fur hosts green algae; this may be a symbiotic relation that affords camouflage to the sloths.[108]
Camouflage is a powerful influence in a large number of mammals, as it helps to conceal individuals from predators or prey.[109] In arctic and subarctic mammals such as the arctic fox (Alopex lagopus), collared lemming (Dicrostonyx groenlandicus), stoat (Mustela erminea), and snowshoe hare (Lepus americanus), seasonal color change between brown in summer and white in winter is driven largely by camouflage.[110] Some arboreal mammals, notably primates and marsupials, have shades of violet, green, or blue skin on parts of their bodies, indicating some distinct advantage in their largely arboreal habitat due to convergent evolution.[107]
Aposematism, warning off possible predators, is the most likely explanation of the black-and-white pelage of many mammals which are able to defend themselves, such as in the foul-smelling skunk and the powerful and aggressive honey badger.[111] Coat color is sometimes sexually dimorphic, as in many primate species.[112] Differences in female and male coat color may indicate nutrition and hormone levels, important in mate selection.[113] Coat color may influence the ability to retain heat, depending on how much light is reflected. Mammals with a darker colored coat can absorb more heat from solar radiation, and stay warmer, and some smaller mammals, such as voles, have darker fur in the winter. The white, pigmentless fur of arctic mammals, such as the polar bear, may reflect more solar radiation directly onto the skin.[85]:166–167[104] The dazzling black-and-white striping of zebras appear to provide some protection from biting flies.[114]
Reproductive system

In male placentals, the penis is used both for urination and copulation. Depending on the species, an erection may be fueled by blood flow into vascular, spongy tissue or by muscular action. A penis may be contained in a prepuce when not erect, and some placentals also have a penis bone (baculum).[115] Marsupials typically have forked penises,[116] while the echidna penis generally has four heads with only two functioning.[117] The testes of most mammals descend into the scrotum which is typically posterior to the penis but is often anterior in marsupials. Female mammals generally have a clitoris, labia majora and labia minora on the outside, while the internal system contains paired oviducts, 1-2 uteri, 1-2 cervices and a vagina. Marsupials have two lateral vaginas and a medial vagina. The "vagina" of monotremes is better understood as a "urogenital sinus". The uterine systems of placental mammals can vary between a duplex, were there are two uteri and cervices which open into the vagina, a bipartite, were two uterine horns have a single cervix that connects to the vagina, a bicornuate, which consists where two uterine horns that are connected distally but separate medially creating a Y-shape, and a simplex, which has a single uterus.[118][119][85]:220–221, 247

The ancestral condition for mammal reproduction is the birthing of relatively undeveloped, either through direct vivipary or a short period as soft-shelled eggs. This is likely due to the fact that the torso could not expand due to the presence of epipubic bones. The oldest demonstration of this reproductive style is with Kayentatherium, which produced undeveloped perinates, but at much higher litter sizes than any modern mammal, 38 specimens.[120] Most modern mammals are viviparous, giving birth to live young. However, the five species of monotreme, the platypus and the four species of echidna, lay eggs. The monotremes have a sex determination system different from that of most other mammals.[121] In particular, the sex chromosomes of a platypus are more like those of a chicken than those of a therian mammal.[122]
Viviparous mammals are in the subclass Theria; those living today are in the marsupial and placental infraclasses. Marsupials have a short gestation period, typically shorter than its estrous cycle and gives birth to an undeveloped newborn that then undergoes further development; in many species, this takes place within a pouch-like sac, the marsupium, located in the front of the mother's abdomen. This is the plesiomorphic condition among viviparous mammals; the presence of epipubic bones in all non-placental mammals prevents the expansion of the torso needed for full pregnancy.[76] Even non-placental eutherians probably reproduced this way.[123] The placentals give birth to relatively complete and developed young, usually after long gestation periods.[124] They get their name from the placenta, which connects the developing fetus to the uterine wall to allow nutrient uptake.[125] In placental mammals, the epipubic is either completely lost or converted into the baculum; allowing the torso to be able to expand and thus birth developed offspring.[120]
The mammary glands of mammals are specialized to produce milk, the primary source of nutrition for newborns. The monotremes branched early from other mammals and do not have the nipples seen in most mammals, but they do have mammary glands. The young lick the milk from a mammary patch on the mother's belly.[126] Compared to placental mammals, the milk of marsupials changes greatly in both production rate and in nutrient composition, due to the underdeveloped young. In addition, the mammary glands have more autonomy allowing them to supply separate milks to young at different development stages.[127] Lactose is the main sugar in placental mammal milk while monotreme and marsupial milk is dominated by oligosaccharides.[128] Weaning is the process in which a mammal becomes less dependent on their mother's milk and more on solid food.[129]
Endothermy
Nearly all mammals are endothermic ("warm-blooded"). Most mammals also have hair to help keep them warm. Like birds, mammals can forage or hunt in weather and climates too cold for ectothermic ("cold-blooded") reptiles and insects. Endothermy requires plenty of food energy, so mammals eat more food per unit of body weight than most reptiles.[130] Small insectivorous mammals eat prodigious amounts for their size. A rare exception, the naked mole-rat produces little metabolic heat, so it is considered an operational poikilotherm.[131] Birds are also endothermic, so endothermy is not unique to mammals.[132]
Species lifespan
Among mammals, species maximum lifespan varies significantly (for example the shrew has a lifespan of two years, whereas the oldest bowhead whale is recorded to be 211 years).[133] Although the underlying basis for these lifespan differences is still uncertain, numerous studies indicate that the ability to repair DNA damage is an important determinant of mammalian lifespan. In a 1974 study by Hart and Setlow,[134] it was found that DNA excision repair capability increased systematically with species lifespan among seven mammalian species. Species lifespan was observed to be robustly correlated with the capacity to recognize DNA double-strand breaks as well as the level of the DNA repair protein Ku80.[133] In a study of the cells from sixteen mammalian species, genes employed in DNA repair were found to be up-regulated in the longer-lived species.[135] The cellular level of the DNA repair enzyme poly ADP ribose polymerase was found to correlate with species lifespan in a study of 13 mammalian species.[136] Three additional studies of a variety of mammalian species also reported a correlation between species lifespan and DNA repair capability.[137][138][139]
Locomotion
Terrestrial

Most vertebrates—the amphibians, the reptiles and some mammals such as humans and bears—are plantigrade, walking on the whole of the underside of the foot. Many mammals, such as cats and dogs, are digitigrade, walking on their toes, the greater stride length allowing more speed. Digitigrade mammals are also often adept at quiet movement.[140] Some animals such as horses are unguligrade, walking on the tips of their toes. This even further increases their stride length and thus their speed.[141] A few mammals, namely the great apes, are also known to walk on their knuckles, at least for their front legs. Giant anteaters[142] and platypuses[143] are also knuckle-walkers. Some mammals are bipeds, using only two limbs for locomotion, which can be seen in, for example, humans and the great apes. Bipedal species have a larger field of vision than quadrupeds, conserve more energy and have the ability to manipulate objects with their hands, which aids in foraging. Instead of walking, some bipeds hop, such as kangaroos and kangaroo rats.[144][145]
Animals will use different gaits for different speeds, terrain and situations. For example, horses show four natural gaits, the slowest horse gait is the walk, then there are three faster gaits which, from slowest to fastest, are the trot, the canter and the gallop. Animals may also have unusual gaits that are used occasionally, such as for moving sideways or backwards. For example, the main human gaits are bipedal walking and running, but they employ many other gaits occasionally, including a four-legged crawl in tight spaces.[146] Mammals show a vast range of gaits, the order that they place and lift their appendages in locomotion. Gaits can be grouped into categories according to their patterns of support sequence. For quadrupeds, there are three main categories: walking gaits, running gaits and leaping gaits.[147] Walking is the most common gait, where some feet are on the ground at any given time, and found in almost all legged animals. Running is considered to occur when at some points in the stride all feet are off the ground in a moment of suspension.[146]
Arboreal
.jpg.webp)
Arboreal animals frequently have elongated limbs that help them cross gaps, reach fruit or other resources, test the firmness of support ahead and, in some cases, to brachiate (swing between trees).[148] Many arboreal species, such as tree porcupines, silky anteaters, spider monkeys, and possums, use prehensile tails to grasp branches. In the spider monkey, the tip of the tail has either a bare patch or adhesive pad, which provides increased friction. Claws can be used to interact with rough substrates and reorient the direction of forces the animal applies. This is what allows squirrels to climb tree trunks that are so large to be essentially flat from the perspective of such a small animal. However, claws can interfere with an animal's ability to grasp very small branches, as they may wrap too far around and prick the animal's own paw. Frictional gripping is used by primates, relying upon hairless fingertips. Squeezing the branch between the fingertips generates frictional force that holds the animal's hand to the branch. However, this type of grip depends upon the angle of the frictional force, thus upon the diameter of the branch, with larger branches resulting in reduced gripping ability. To control descent, especially down large diameter branches, some arboreal animals such as squirrels have evolved highly mobile ankle joints that permit rotating the foot into a 'reversed' posture. This allows the claws to hook into the rough surface of the bark, opposing the force of gravity. Small size provides many advantages to arboreal species: such as increasing the relative size of branches to the animal, lower center of mass, increased stability, lower mass (allowing movement on smaller branches) and the ability to move through more cluttered habitat.[148] Size relating to weight affects gliding animals such as the sugar glider.[149] Some species of primate, bat and all species of sloth achieve passive stability by hanging beneath the branch. Both pitching and tipping become irrelevant, as the only method of failure would be losing their grip.[148]
Aerial
Bats are the only mammals that can truly fly. They fly through the air at a constant speed by moving their wings up and down (usually with some fore-aft movement as well). Because the animal is in motion, there is some airflow relative to its body which, combined with the velocity of the wings, generates a faster airflow moving over the wing. This generates a lift force vector pointing forwards and upwards, and a drag force vector pointing rearwards and upwards. The upwards components of these counteract gravity, keeping the body in the air, while the forward component provides thrust to counteract both the drag from the wing and from the body as a whole.[150]
The wings of bats are much thinner and consist of more bones than those of birds, allowing bats to maneuver more accurately and fly with more lift and less drag.[151][152] By folding the wings inwards towards their body on the upstroke, they use 35% less energy during flight than birds.[153] The membranes are delicate, ripping easily; however, the tissue of the bat's membrane is able to regrow, such that small tears can heal quickly.[154] The surface of their wings is equipped with touch-sensitive receptors on small bumps called Merkel cells, also found on human fingertips. These sensitive areas are different in bats, as each bump has a tiny hair in the center, making it even more sensitive and allowing the bat to detect and collect information about the air flowing over its wings, and to fly more efficiently by changing the shape of its wings in response.[155]
Fossorial and subterranean
A fossorial (from Latin fossor, meaning "digger") is an animal adapted to digging which lives primarily, but not solely, underground. Some examples are badgers, and naked mole-rats. Many rodent species are also considered fossorial because they live in burrows for most but not all of the day. Species that live exclusively underground are subterranean, and those with limited adaptations to a fossorial lifestyle sub-fossorial. Some organisms are fossorial to aid in temperature regulation while others use the underground habitat for protection from predators or for food storage.[156]
Fossorial mammals have a fusiform body, thickest at the shoulders and tapering off at the tail and nose. Unable to see in the dark burrows, most have degenerated eyes, but degeneration varies between species; pocket gophers, for example, are only semi-fossorial and have very small yet functional eyes, in the fully fossorial marsupial mole the eyes are degenerated and useless, talpa moles have vestigial eyes and the cape golden mole has a layer of skin covering the eyes. External ears flaps are also very small or absent. Truly fossorial mammals have short, stout legs as strength is more important than speed to a burrowing mammal, but semi-fossorial mammals have cursorial legs. The front paws are broad and have strong claws to help in loosening dirt while excavating burrows, and the back paws have webbing, as well as claws, which aids in throwing loosened dirt backwards. Most have large incisors to prevent dirt from flying into their mouth.[157]
Many fossorial mammals such as shrews, hedgehogs, and moles were classified under the now obsolete order Insectivora.[158]
Aquatic
Fully aquatic mammals, the cetaceans and sirenians, have lost their legs and have a tail fin to propel themselves through the water. Flipper movement is continuous. Whales swim by moving their tail fin and lower body up and down, propelling themselves through vertical movement, while their flippers are mainly used for steering. Their skeletal anatomy allows them to be fast swimmers. Most species have a dorsal fin to prevent themselves from turning upside-down in the water.[159][160] The flukes of sirenians are raised up and down in long strokes to move the animal forward, and can be twisted to turn. The forelimbs are paddle-like flippers which aid in turning and slowing.[161]
Semi-aquatic mammals, like pinnipeds, have two pairs of flippers on the front and back, the fore-flippers and hind-flippers. The elbows and ankles are enclosed within the body.[162][163] Pinnipeds have several adaptions for reducing drag. In addition to their streamlined bodies, they have smooth networks of muscle bundles in their skin that may increase laminar flow and make it easier for them to slip through water. They also lack arrector pili, so their fur can be streamlined as they swim.[164] They rely on their fore-flippers for locomotion in a wing-like manner similar to penguins and sea turtles.[165] Fore-flipper movement is not continuous, and the animal glides between each stroke.[163] Compared to terrestrial carnivorans, the fore-limbs are reduced in length, which gives the locomotor muscles at the shoulder and elbow joints greater mechanical advantage;[162] the hind-flippers serve as stabilizers.[164] Other semi-aquatic mammals include beavers, hippopotamuses, otters and platypuses.[166] Hippos are very large semi-aquatic mammals, and their barrel-shaped bodies have graviportal skeletal structures,[167] adapted to carrying their enormous weight, and their specific gravity allows them to sink and move along the bottom of a river.[168]
Behavior
Communication and vocalization

Many mammals communicate by vocalizing. Vocal communication serves many purposes, including in mating rituals, as warning calls,[170] to indicate food sources, and for social purposes. Males often call during mating rituals to ward off other males and to attract females, as in the roaring of lions and red deer.[171] The songs of the humpback whale may be signals to females;[172] they have different dialects in different regions of the ocean.[173] Social vocalizations include the territorial calls of gibbons, and the use of frequency in greater spear-nosed bats to distinguish between groups.[174] The vervet monkey gives a distinct alarm call for each of at least four different predators, and the reactions of other monkeys vary according to the call. For example, if an alarm call signals a python, the monkeys climb into the trees, whereas the eagle alarm causes monkeys to seek a hiding place on the ground.[169] Prairie dogs similarly have complex calls that signal the type, size, and speed of an approaching predator.[175] Elephants communicate socially with a variety of sounds including snorting, screaming, trumpeting, roaring and rumbling. Some of the rumbling calls are infrasonic, below the hearing range of humans, and can be heard by other elephants up to 6 miles (9.7 km) away at still times near sunrise and sunset.[176]
Mammals signal by a variety of means. Many give visual anti-predator signals, as when deer and gazelle stot, honestly indicating their fit condition and their ability to escape,[177][178] or when white-tailed deer and other prey mammals flag with conspicuous tail markings when alarmed, informing the predator that it has been detected.[179] Many mammals make use of scent-marking, sometimes possibly to help defend territory, but probably with a range of functions both within and between species.[180][181][182] Microbats and toothed whales including oceanic dolphins vocalize both socially and in echolocation.[183][184][185]
Feeding
To maintain a high constant body temperature is energy expensive—mammals therefore need a nutritious and plentiful diet. While the earliest mammals were probably predators, different species have since adapted to meet their dietary requirements in a variety of ways. Some eat other animals—this is a carnivorous diet (and includes insectivorous diets). Other mammals, called herbivores, eat plants, which contain complex carbohydrates such as cellulose. An herbivorous diet includes subtypes such as granivory (seed eating), folivory (leaf eating), frugivory (fruit eating), nectarivory (nectar eating), gummivory (gum eating) and mycophagy (fungus eating). The digestive tract of an herbivore is host to bacteria that ferment these complex substances, and make them available for digestion, which are either housed in the multichambered stomach or in a large cecum.[89] Some mammals are coprophagous, consuming feces to absorb the nutrients not digested when the food was first ingested.[85]:131–137 An omnivore eats both prey and plants. Carnivorous mammals have a simple digestive tract because the proteins, lipids and minerals found in meat require little in the way of specialized digestion. Exceptions to this include baleen whales who also house gut flora in a multi-chambered stomach, like terrestrial herbivores.[186]
The size of an animal is also a factor in determining diet type (Allen's rule). Since small mammals have a high ratio of heat-losing surface area to heat-generating volume, they tend to have high energy requirements and a high metabolic rate. Mammals that weigh less than about 18 ounces (510 g; 1.1 lb) are mostly insectivorous because they cannot tolerate the slow, complex digestive process of an herbivore. Larger animals, on the other hand, generate more heat and less of this heat is lost. They can therefore tolerate either a slower collection process (carnivores that feed on larger vertebrates) or a slower digestive process (herbivores).[187] Furthermore, mammals that weigh more than 18 ounces (510 g; 1.1 lb) usually cannot collect enough insects during their waking hours to sustain themselves. The only large insectivorous mammals are those that feed on huge colonies of insects (ants or termites).[188]

_scavenging_a_narwhal_whale_(Monodon_monoceros)_carcass_-_journal.pone.0060797.g001-A.png.webp)
Some mammals are omnivores and display varying degrees of carnivory and herbivory, generally leaning in favor of one more than the other. Since plants and meat are digested differently, there is a preference for one over the other, as in bears where some species may be mostly carnivorous and others mostly herbivorous.[190] They are grouped into three categories: mesocarnivory (50–70% meat), hypercarnivory (70% and greater of meat), and hypocarnivory (50% or less of meat). The dentition of hypocarnivores consists of dull, triangular carnassial teeth meant for grinding food. Hypercarnivores, however, have conical teeth and sharp carnassials meant for slashing, and in some cases strong jaws for bone-crushing, as in the case of hyenas, allowing them to consume bones; some extinct groups, notably the Machairodontinae, had saber-shaped canines.[189]
Some physiological carnivores consume plant matter and some physiological herbivores consume meat. From a behavioral aspect, this would make them omnivores, but from the physiological standpoint, this may be due to zoopharmacognosy. Physiologically, animals must be able to obtain both energy and nutrients from plant and animal materials to be considered omnivorous. Thus, such animals are still able to be classified as carnivores and herbivores when they are just obtaining nutrients from materials originating from sources that do not seemingly complement their classification.[191] For example, it is well documented that some ungulates such as giraffes, camels, and cattle, will gnaw on bones to consume particular minerals and nutrients.[192] Also, cats, which are generally regarded as obligate carnivores, occasionally eat grass to regurgitate indigestible material (such as hairballs), aid with hemoglobin production, and as a laxative.[193]
Many mammals, in the absence of sufficient food requirements in an environment, suppress their metabolism and conserve energy in a process known as hibernation.[194] In the period preceding hibernation, larger mammals, such as bears, become polyphagic to increase fat stores, whereas smaller mammals prefer to collect and stash food.[195] The slowing of the metabolism is accompanied by a decreased heart and respiratory rate, as well as a drop in internal temperatures, which can be around ambient temperature in some cases. For example, the internal temperatures of hibernating arctic ground squirrels can drop to −2.9 °C (26.8 °F), however the head and neck always stay above 0 °C (32 °F).[196] A few mammals in hot environments aestivate in times of drought or extreme heat, for example the fat-tailed dwarf lemur (Cheirogaleus medius).[197]
Intelligence
In intelligent mammals, such as primates, the cerebrum is larger relative to the rest of the brain. Intelligence itself is not easy to define, but indications of intelligence include the ability to learn, matched with behavioral flexibility. Rats, for example, are considered to be highly intelligent, as they can learn and perform new tasks, an ability that may be important when they first colonize a fresh habitat. In some mammals, food gathering appears to be related to intelligence: a deer feeding on plants has a brain smaller than a cat, which must think to outwit its prey.[188]
Tool use by animals may indicate different levels of learning and cognition. The sea otter uses rocks as essential and regular parts of its foraging behaviour (smashing abalone from rocks or breaking open shells), with some populations spending 21% of their time making tools.[198] Other tool use, such as chimpanzees using twigs to "fish" for termites, may be developed by watching others use tools and may even be a true example of animal teaching.[199] Tools may even be used in solving puzzles in which the animal appears to experience a "Eureka moment".[200] Other mammals that do not use tools, such as dogs, can also experience a Eureka moment.[201]
Brain size was previously considered a major indicator of the intelligence of an animal. Since most of the brain is used for maintaining bodily functions, greater ratios of brain to body mass may increase the amount of brain mass available for more complex cognitive tasks. Allometric analysis indicates that mammalian brain size scales at approximately the 2⁄3 or 3⁄4 exponent of the body mass. Comparison of a particular animal's brain size with the expected brain size based on such allometric analysis provides an encephalisation quotient that can be used as another indication of animal intelligence.[202] Sperm whales have the largest brain mass of any animal on earth, averaging 8,000 cubic centimetres (490 in3) and 7.8 kilograms (17 lb) in mature males.[203]
Self-awareness appears to be a sign of abstract thinking. Self-awareness, although not well-defined, is believed to be a precursor to more advanced processes such as metacognitive reasoning. The traditional method for measuring this is the mirror test, which determines if an animal possesses the ability of self-recognition.[204] Mammals that have passed the mirror test include Asian elephants (some pass, some do not);[205] chimpanzees;[206] bonobos;[207] orangutans;[208] humans, from 18 months (mirror stage);[209] bottlenose dolphins[lower-alpha 1][210] killer whales;[211] and false killer whales.[211]
Social structure


Eusociality is the highest level of social organization. These societies have an overlap of adult generations, the division of reproductive labor and cooperative caring of young. Usually insects, such as bees, ants and termites, have eusocial behavior, but it is demonstrated in two rodent species: the naked mole-rat[212] and the Damaraland mole-rat.[213]
Presociality is when animals exhibit more than just sexual interactions with members of the same species, but fall short of qualifying as eusocial. That is, presocial animals can display communal living, cooperative care of young, or primitive division of reproductive labor, but they do not display all of the three essential traits of eusocial animals. Humans and some species of Callitrichidae (marmosets and tamarins) are unique among primates in their degree of cooperative care of young.[214] Harry Harlow set up an experiment with rhesus monkeys, presocial primates, in 1958; the results from this study showed that social encounters are necessary in order for the young monkeys to develop both mentally and sexually.[215]
A fission-fusion society is a society that changes frequently in its size and composition, making up a permanent social group called the "parent group". Permanent social networks consist of all individual members of a community and often varies to track changes in their environment. In a fission–fusion society, the main parent group can fracture (fission) into smaller stable subgroups or individuals to adapt to environmental or social circumstances. For example, a number of males may break off from the main group in order to hunt or forage for food during the day, but at night they may return to join (fusion) the primary group to share food and partake in other activities. Many mammals exhibit this, such as primates (for example orangutans and spider monkeys),[216] elephants,[217] spotted hyenas,[218] lions,[219] and dolphins.[220]
Solitary animals defend a territory and avoid social interactions with the members of its species, except during breeding season. This is to avoid resource competition, as two individuals of the same species would occupy the same niche, and to prevent depletion of food.[221] A solitary animal, while foraging, can also be less conspicuous to predators or prey.[222]

_under_the_rain_(14027667943).jpg.webp)
In a hierarchy, individuals are either dominant or submissive. A despotic hierarchy is where one individual is dominant while the others are submissive, as in wolves and lemurs,[223] and a pecking order is a linear ranking of individuals where there is a top individual and a bottom individual. Pecking orders may also be ranked by sex, where the lowest individual of a sex has a higher ranking than the top individual of the other sex, as in hyenas.[224] Dominant individuals, or alphas, have a high chance of reproductive success, especially in harems where one or a few males (resident males) have exclusive breeding rights to females in a group.[225] Non-resident males can also be accepted in harems, but some species, such as the common vampire bat (Desmodus rotundus), may be more strict.[226]
Some mammals are perfectly monogamous, meaning that they mate for life and take no other partners (even after the original mate's death), as with wolves, Eurasian beavers, and otters.[227][228] There are three types of polygamy: either one or multiple dominant males have breeding rights (polygyny), multiple males that females mate with (polyandry), or multiple males have exclusive relations with multiple females (polygynandry). It is much more common for polygynous mating to happen, which, excluding leks, are estimated to occur in up to 90% of mammals.[229] Lek mating occurs when males congregate around females and try to attract them with various courtship displays and vocalizations, as in harbor seals.[230]
All higher mammals (excluding monotremes) share two major adaptations for care of the young: live birth and lactation. These imply a group-wide choice of a degree of parental care. They may build nests and dig burrows to raise their young in, or feed and guard them often for a prolonged period of time. Many mammals are K-selected, and invest more time and energy into their young than do r-selected animals. When two animals mate, they both share an interest in the success of the offspring, though often to different extremes. Mammalian females exhibit some degree of maternal aggression, another example of parental care, which may be targeted against other females of the species or the young of other females; however, some mammals may "aunt" the infants of other females, and care for them. Mammalian males may play a role in child rearing, as with tenrecs, however this varies species to species, even within the same genus. For example, the males of the southern pig-tailed macaque (Macaca nemestrina) do not participate in child care, whereas the males of the Japanese macaque (M. fuscata) do.[231]
Humans and other mammals
In human culture

Non-human mammals play a wide variety of roles in human culture. They are the most popular of pets, with tens of millions of dogs, cats and other animals including rabbits and mice kept by families around the world.[232][233][234] Mammals such as mammoths, horses and deer are among the earliest subjects of art, being found in Upper Paleolithic cave paintings such as at Lascaux.[235] Major artists such as Albrecht Dürer, George Stubbs and Edwin Landseer are known for their portraits of mammals.[236] Many species of mammals have been hunted for sport and for food; deer and wild boar are especially popular as game animals.[237][238][239] Mammals such as horses and dogs are widely raced for sport, often combined with betting on the outcome.[240][241] There is a tension between the role of animals as companions to humans, and their existence as individuals with rights of their own.[242] Mammals further play a wide variety of roles in literature,[243][244][245] film,[246] mythology, and religion.[247][248][249]
Uses and importance

Domestic mammals form a large part of the livestock raised for meat across the world. They include (2009) around 1.4 billion cattle, 1 billion sheep, 1 billion domestic pigs,[250][251] and (1985) over 700 million rabbits.[252] Working domestic animals including cattle and horses have been used for work and transport from the origins of agriculture, their numbers declining with the arrival of mechanised transport and agricultural machinery. In 2004 they still provided some 80% of the power for the mainly small farms in the third world, and some 20% of the world's transport, again mainly in rural areas. In mountainous regions unsuitable for wheeled vehicles, pack animals continue to transport goods.[253] Mammal skins provide leather for shoes, clothing and upholstery.[254] Wool from mammals including sheep, goats and alpacas has been used for centuries for clothing.[255][256] Mammals serve a major role in science as experimental animals, both in fundamental biological research, such as in genetics,[257] and in the development of new medicines, which must be tested exhaustively to demonstrate their safety.[258] Millions of mammals, especially mice and rats, are used in experiments each year.[259] A knockout mouse is a genetically modified mouse with an inactivated gene, replaced or disrupted with an artificial piece of DNA. They enable the study of sequenced genes whose functions are unknown.[260] A small percentage of the mammals are non-human primates, used in research for their similarity to humans.[261][262][263]
Charles Darwin, Jared Diamond and others have noted the importance of domesticated mammals in the Neolithic development of agriculture and of civilization, causing farmers to replace hunter-gatherers around the world.[lower-alpha 2][265] This transition from hunting and gathering to herding flocks and growing crops was a major step in human history. The new agricultural economies, based on domesticated mammals, caused "radical restructuring of human societies, worldwide alterations in biodiversity, and significant changes in the Earth's landforms and its atmosphere... momentous outcomes".[266]
Hybrids


Hybrids are offspring resulting from the breeding of two genetically distinct individuals, which usually will result in a high degree of heterozygosity, though hybrid and heterozygous are not synonymous. The deliberate or accidental hybridizing of two or more species of closely related animals through captive breeding is a human activity which has been in existence for millennia and has grown for economic purposes.[267] Hybrids between different subspecies within a species (such as between the Bengal tiger and Siberian tiger) are known as intra-specific hybrids. Hybrids between different species within the same genus (such as between lions and tigers) are known as interspecific hybrids or crosses. Hybrids between different genera (such as between sheep and goats) are known as intergeneric hybrids.[268] Natural hybrids will occur in hybrid zones, where two populations of species within the same genera or species living in the same or adjacent areas will interbreed with each other. Some hybrids have been recognized as species, such as the red wolf (though this is controversial).[269]
Artificial selection, the deliberate selective breeding of domestic animals, is being used to breed back recently extinct animals in an attempt to achieve an animal breed with a phenotype that resembles that extinct wildtype ancestor. A breeding-back (intraspecific) hybrid may be very similar to the extinct wildtype in appearance, ecological niche and to some extent genetics, but the initial gene pool of that wild type is lost forever with its extinction. As a result, bred-back breeds are at best vague look-alikes of extinct wildtypes, as Heck cattle are of the aurochs.[270]
Purebred wild species evolved to a specific ecology can be threatened with extinction[271] through the process of genetic pollution, the uncontrolled hybridization, introgression genetic swamping which leads to homogenization or out-competition from the heterosic hybrid species.[272] When new populations are imported or selectively bred by people, or when habitat modification brings previously isolated species into contact, extinction in some species, especially rare varieties, is possible.[273] Interbreeding can swamp the rarer gene pool and create hybrids, depleting the purebred gene pool. For example, the endangered wild water buffalo is most threatened with extinction by genetic pollution from the domestic water buffalo. Such extinctions are not always apparent from a morphological standpoint. Some degree of gene flow is a normal evolutionary process, nevertheless, hybridization threatens the existence of rare species.[274][275]
Threats
The loss of species from ecological communities, defaunation, is primarily driven by human activity.[276] This has resulted in empty forests, ecological communities depleted of large vertebrates.[277][278] In the Quaternary extinction event, the mass die-off of megafaunal variety coincided with the appearance of humans, suggesting a human influence. One hypothesis is that humans hunted large mammals, such as the woolly mammoth, into extinction.[279][280] The 2019 Global Assessment Report on Biodiversity and Ecosystem Services by IPBES states that the total biomass of wild mammals has declined by 82 percent since the beginning of human civilization.[281][282] Wild animals make up just 4% of mammalian biomass on earth, while humans and their domesticated animals make up 96%.[283]
Various species are predicted to become extinct in the near future,[284] among them the rhinoceros,[285] primates,[286] pangolins,[287] and giraffes.[288] According to the WWF's 2020 Living Planet Report, vertebrate wildlife populations have declined by 68% since 1970 as a result of human activities, particularly overconsumption, population growth and intensive farming, which is evidence that humans have triggered a sixth mass extinction event.[289][290] Hunting alone threatens hundreds of mammalian species around the world.[291][292] Scientists claim that the growing demand for meat is contributing to biodiversity loss as this is a significant driver of deforestation and habitat destruction; species-rich habitats, such as significant portions of the Amazon rainforest, are being converted to agricultural land for meat production.[293][294][295] Another influence is over-hunting and poaching, which can reduce the overall population of game animals,[296] especially those located near villages,[297] as in the case of peccaries.[298] The effects of poaching can especially be seen in the ivory trade with African elephants. Marine mammals are at risk from entanglement from fishing gear, notably cetaceans, with discard mortalities ranging from 65,000 to 86,000 individuals annually.[299]
Attention is being given to endangered species globally, notably through the Convention on Biological Diversity, otherwise known as the Rio Accord, which includes 189 signatory countries that are focused on identifying endangered species and habitats.[300] Another notable conservation organization is the IUCN, which has a membership of over 1,200 governmental and non-governmental organizations.[301]
Recent extinctions can be directly attributed to human influences.[302][276] The IUCN characterizes 'recent' extinction as those that have occurred past the cut-off point of 1500,[303] and around 80 mammal species have gone extinct since that time and 2015.[304] Some species, such as the Père David's deer[305] are extinct in the wild, and survive solely in captive populations. Other species, such as the Florida panther, are ecologically extinct, surviving in such low numbers that they essentially have no impact on the ecosystem.[306]:318 Other populations are only locally extinct (extirpated), still existing elsewhere, but reduced in distribution,[306]:75–77 as with the extinction of gray whales in the Atlantic.[307]
Notes
- Decreased latency to approach the mirror, repetitious head circling and close viewing of the marked areas were considered signs of self-recognition since they do not have arms and cannot touch the marked areas.[210]
- Diamond discussed this matter further in his 1997 book Guns, Germs, and Steel.[264]
See also
- List of mammal genera – living mammals
- List of mammalogists
- List of monotremes and marsupials
- List of placental mammals
- List of prehistoric mammals
- List of threatened mammals of the United States
- Lists of mammals by population size
- Lists of mammals by region
- Mammals described in the 2000s
- Mammals in culture
References
- Vaughan TA, Ryan JM, Czaplewski NJ (2013). "Classification of Mammals". Mammalogy (6 ed.). Jones and Bartlett Learning. ISBN 978-1-284-03209-3.
- Simpson, George Gaylord (1945). "Principles of classification, and a classification of mammals". Bulletin of the American Museum of Natural History. 85.
- Szalay FS (1999). "Classification of mammals above the species level: Review". Journal of Vertebrate Paleontology. 19 (1): 191–195. doi:10.1080/02724634.1999.10011133. JSTOR 4523980.
- Wilson D, Reeder D, eds. (2005). "Preface and introductory material". Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. p. xxvi. ISBN 978-0-8018-8221-0. OCLC 62265494.
- "Mammals". The IUCN Red List of Threatened Species. International Union for Conservation of Nature (IUCN). April 2010. Retrieved 23 August 2016.
- Burgin CJ, Colella JP, Kahn PL, Upham NS (1 February 2018). "How many species of mammals are there?". Journal of Mammalogy. 99 (1): 1–14. doi:10.1093/jmammal/gyx147.
- Rowe T (1988). "Definition, diagnosis, and origin of Mammalia" (PDF). Journal of Vertebrate Paleontology. 8 (3): 241–264. doi:10.1080/02724634.1988.10011708.
- Lyell C (1871). The Student's Elements of Geology. London, UK: John Murray. p. 347. ISBN 978-1-345-18248-4.
- Cifelli RL, Davis BM (December 2003). "Paleontology. Marsupial origins". Science. 302 (5652): 1899–900. doi:10.1126/science.1092272. PMID 14671280.
- Kemp TS (2005). The Origin and Evolution of Mammals (PDF). United Kingdom: Oxford University Press. p. 3. ISBN 978-0-19-850760-4. OCLC 232311794.
- Datta PM (2005). "Earliest mammal with transversely expanded upper molar from the Late Triassic (Carnian) Tiki Formation, South Rewa Gondwana Basin, India". Journal of Vertebrate Paleontology. 25 (1): 200–207. doi:10.1671/0272-4634(2005)025[0200:EMWTEU]2.0.CO;2.
- Luo Z, Martin T (2007). "Analysis of Molar Structure and Phylogeny of Docodont Genera" (PDF). Bulletin of Carnegie Museum of Natural History. 39: 27–47. doi:10.2992/0145-9058(2007)39[27:AOMSAP]2.0.CO;2. Archived from the original (PDF) on 3 March 2016. Retrieved 8 April 2013.
- McKenna MC, Bell SG (1997). Classification of Mammals above the Species Level. New York, NY: Columbia University Press. ISBN 978-0-231-11013-6. OCLC 37345734.
- Nilsson MA, Churakov G, Sommer M, Tran NV, Zemann A, Brosius J, Schmitz J (July 2010). "Tracking marsupial evolution using archaic genomic retroposon insertions". PLOS Biology. 8 (7): e1000436. doi:10.1371/journal.pbio.1000436. PMC 2910653. PMID 20668664.
- Kriegs JO, Churakov G, Kiefmann M, Jordan U, Brosius J, Schmitz J (April 2006). "Retroposed elements as archives for the evolutionary history of placental mammals". PLOS Biology. 4 (4): e91. doi:10.1371/journal.pbio.0040091. PMC 1395351. PMID 16515367.
- Nishihara H, Maruyama S, Okada N (March 2009). "Retroposon analysis and recent geological data suggest near-simultaneous divergence of the three superorders of mammals". Proceedings of the National Academy of Sciences of the United States of America. 106 (13): 5235–40. Bibcode:2009PNAS..106.5235N. doi:10.1073/pnas.0809297106. PMC 2655268. PMID 19286970.
- Springer MS, Murphy WJ, Eizirik E, O'Brien SJ (February 2003). "Placental mammal diversification and the Cretaceous-Tertiary boundary". Proceedings of the National Academy of Sciences of the United States of America. 100 (3): 1056–61. Bibcode:2003PNAS..100.1056S. doi:10.1073/pnas.0334222100. PMC 298725. PMID 12552136.
- Tarver JE, Dos Reis M, Mirarab S, Moran RJ, Parker S, O'Reilly JE, et al. (January 2016). "The Interrelationships of Placental Mammals and the Limits of Phylogenetic Inference". Genome Biology and Evolution. 8 (2): 330–44. doi:10.1093/gbe/evv261. hdl:1983/64d6e437-3320-480d-a16c-2e5b2e6b61d4. PMC 4779606. PMID 26733575.
- Springer MS, Meredith RW, Janecka JE, Murphy WJ (September 2011). "The historical biogeography of Mammalia". Philosophical Transactions of the Royal Society of London B. 366 (1577): 2478–502. doi:10.1098/rstb.2011.0023. PMC 3138613. PMID 21807730.
- Meng J, Wang Y, Li C (April 2011). "Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont". Nature. 472 (7342): 181–5. Bibcode:2011Natur.472..181M. doi:10.1038/nature09921. PMID 21490668. S2CID 4428972.
- Ahlberg PE, Milner AR (April 1994). "The Origin and Early Diversification of Tetrapods". Nature. 368 (6471): 507–514. Bibcode:1994Natur.368..507A. doi:10.1038/368507a0. S2CID 4369342.
- "Amniota – Palaeos". Archived from the original on 2010-12-20.
- "Synapsida overview – Palaeos". Archived from the original on 2010-12-20.
- Kemp TS (July 2006). "The origin and early radiation of the therapsid mammal-like reptiles: a palaeobiological hypothesis" (PDF). Journal of Evolutionary Biology. 19 (4): 1231–47. doi:10.1111/j.1420-9101.2005.01076.x. PMID 16780524.
- Bennett AF, Ruben JA (1986). "The metabolic and thermoregulatory status of therapsids". In Hotton III N, MacLean JJ, Roth J, Roth EC (eds.). The ecology and biology of mammal-like reptiles. Washington, DC: Smithsonian Institution Press. pp. 207–218. ISBN 978-0-87474-524-5.
- Kermack DM, Kermack KA (1984). The evolution of mammalian characters. Washington D.C.: Croom Helm. ISBN 978-0-7099-1534-8. OCLC 10710687.
- Tanner LH, Lucas SG, Chapman MG (2004). "Assessing the record and causes of Late Triassic extinctions" (PDF). Earth-Science Reviews. 65 (1–2): 103–139. Bibcode:2004ESRv...65..103T. doi:10.1016/S0012-8252(03)00082-5. Archived from the original on October 25, 2007.CS1 maint: unfit URL (link)
- Brusatte SL, Benton MJ, Ruta M, Lloyd GT (September 2008). "Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs" (PDF). Science. 321 (5895): 1485–8. Bibcode:2008Sci...321.1485B. doi:10.1126/science.1161833. PMID 18787166. S2CID 13393888.
- Gauthier JA (1986). "Saurischian monophyly and the origin of birds". In Padian K (ed.). The Origin of Birds and the Evolution of Flight. Memoirs of the California Academy of Sciences. 8. San Francisco: California Academy of Sciences. pp. 1–55.
- Sereno PC (1991). "Basal archosaurs: phylogenetic relationships and functional implications". Memoirs of the Society of Vertebrate Paleontology. 2: 1–53. doi:10.2307/3889336. JSTOR 3889336.
- MacLeod N, Rawson PF, Forey PL, Banner FT, Boudagher-Fadel MK, Bown PR, et al. (1997). "The Cretaceous–Tertiary biotic transition". Journal of the Geological Society. 154 (2): 265–292. Bibcode:1997JGSoc.154..265M. doi:10.1144/gsjgs.154.2.0265. S2CID 129654916.
- Hunt DM, Hankins MW, Collin SP, Marshall NJ. Evolution of Visual and Non-visual Pigments. London: Springer. p. 73. ISBN 978-1-4614-4354-4. OCLC 892735337.
- Bakalar N (2006). "Jurassic "Beaver" Found; Rewrites History of Mammals". National Geographic News. Retrieved 28 May 2016.
- Hall MI, Kamilar JM, Kirk EC (December 2012). "Eye shape and the nocturnal bottleneck of mammals". Proceedings of the Royal Society B: Biological Sciences. 279 (1749): 4962–8. doi:10.1098/rspb.2012.2258. PMC 3497252. PMID 23097513.
- Luo ZX (December 2007). "Transformation and diversification in early mammal evolution". Nature. 450 (7172): 1011–9. Bibcode:2007Natur.450.1011L. doi:10.1038/nature06277. PMID 18075580. S2CID 4317817.
- Pickrell J (2003). "Oldest Marsupial Fossil Found in China". National Geographic News. Retrieved 28 May 2016.
- Luo ZX, Yuan CX, Meng QJ, Ji Q (August 2011). "A Jurassic eutherian mammal and divergence of marsupials and placentals". Nature. 476 (7361): 442–5. Bibcode:2011Natur.476..442L. doi:10.1038/nature10291. PMID 21866158. S2CID 205225806.
- Ji Q, Luo ZX, Yuan CX, Wible JR, Zhang JP, Georgi JA (April 2002). "The earliest known eutherian mammal". Nature. 416 (6883): 816–22. Bibcode:2002Natur.416..816J. doi:10.1038/416816a. PMID 11976675. S2CID 4330626.
- Novacek MJ, Rougier GW, Wible JR, McKenna MC, Dashzeveg D, Horovitz I (October 1997). "Epipubic bones in eutherian mammals from the late Cretaceous of Mongolia". Nature. 389 (6650): 483–6. Bibcode:1997Natur.389..483N. doi:10.1038/39020. PMID 9333234. S2CID 205026882.
- Power ML, Schulkin J (2012). "Evolution of Live Birth in Mammals". Evolution of the Human Placenta. Baltimore: Johns Hopkins University Press. p. 68. ISBN 978-1-4214-0643-5.
- Rowe T, Rich TH, Vickers-Rich P, Springer M, Woodburne MO (January 2008). "The oldest platypus and its bearing on divergence timing of the platypus and echidna clades". Proceedings of the National Academy of Sciences of the United States of America. 105 (4): 1238–42. Bibcode:2008PNAS..105.1238R. doi:10.1073/pnas.0706385105. PMC 2234122. PMID 18216270.
- Grant T (1995). "Reproduction". The Platypus: A Unique Mammal. Sydney: University of New South Wales. p. 55. ISBN 978-0-86840-143-0. OCLC 33842474.
- Goldman AS (June 2012). "Evolution of immune functions of the mammary gland and protection of the infant". Breastfeeding Medicine. 7 (3): 132–42. doi:10.1089/bfm.2012.0025. PMID 22577734.
- Rose KD (2006). The Beginning of the Age of Mammals. Baltimore: Johns Hopkins University Press. pp. 82–83. ISBN 978-0-8018-8472-6. OCLC 646769601.
- Brink AS (1955). "A study on the skeleton of Diademodon". Palaeontologia Africana. 3: 3–39.
- Kemp TS (1982). Mammal-like reptiles and the origin of mammals. London: Academic Press. p. 363. ISBN 978-0-12-404120-2. OCLC 8613180.
- Estes R (1961). "Cranial anatomy of the cynodont reptile Thrinaxodon liorhinus". Bulletin of the Museum of Comparative Zoology (1253): 165–180.
- "Thrinaxodon: The Emerging Mammal". National Geographic Daily News. February 11, 2009. Retrieved August 26, 2012.
- Bajdek P, Qvarnström M, Owocki K, Sulej T, Sennikov AG, Golubev VK, Niedźwiedzki G (2015). "Microbiota and food residues including possible evidence of pre-mammalian hair in Upper Permian coprolites from Russia". Lethaia. 49 (4): 455–477. doi:10.1111/let.12156.
- Botha-Brink J, Angielczyk KD (2010). "Do extraordinarily high growth rates in Permo-Triassic dicynodonts (Therapsida, Anomodontia) explain their success before and after the end-Permian extinction?". Zoological Journal of the Linnean Society. 160 (2): 341–365. doi:10.1111/j.1096-3642.2009.00601.x.
- Paul GS (1988). Predatory Dinosaurs of the World. New York: Simon and Schuster. p. 464. ISBN 978-0-671-61946-6. OCLC 18350868.
- Watson JM, Graves JA (1988). "Monotreme Cell-Cycles and the Evolution of Homeothermy". Australian Journal of Zoology. 36 (5): 573–584. doi:10.1071/ZO9880573.
- McNab BK (1980). "Energetics and the limits to the temperate distribution in armadillos". Journal of Mammalogy. 61 (4): 606–627. doi:10.2307/1380307. JSTOR 1380307.
- Kielan-Jaworowska Z, Hurum JH (2006). "Limb posture in early mammals: Sprawling or parasagittal" (PDF). Acta Palaeontologica Polonica. 51 (3): 10237–10239.
- Lillegraven JA, Kielan-Jaworowska Z, Clemens WA (1979). Mesozoic Mammals: The First Two-Thirds of Mammalian History. University of California Press. p. 321. ISBN 978-0-520-03951-3. OCLC 5910695.
- Oftedal OT (July 2002). "The mammary gland and its origin during synapsid evolution". Journal of Mammary Gland Biology and Neoplasia. 7 (3): 225–52. doi:10.1023/A:1022896515287. PMID 12751889. S2CID 25806501.
- Oftedal OT (July 2002). "The origin of lactation as a water source for parchment-shelled eggs". Journal of Mammary Gland Biology and Neoplasia. 7 (3): 253–66. doi:10.1023/A:1022848632125. PMID 12751890. S2CID 8319185.
- "Breast Development". Texas Children's Hospital. Archived from the original on 2021-01-13. Retrieved 13 January 2021.
- Sahney S, Benton MJ, Ferry PA (August 2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land". Biology Letters. 6 (4): 544–7. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
- Smith FA, Boyer AG, Brown JH, Costa DP, Dayan T, Ernest SK, et al. (November 2010). "The evolution of maximum body size of terrestrial mammals". Science. 330 (6008): 1216–9. Bibcode:2010Sci...330.1216S. CiteSeerX 10.1.1.383.8581. doi:10.1126/science.1194830. PMID 21109666. S2CID 17272200.
- Simmons NB, Seymour KL, Habersetzer J, Gunnell GF (February 2008). "Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation". Nature. 451 (7180): 818–21. Bibcode:2008Natur.451..818S. doi:10.1038/nature06549. hdl:2027.42/62816. PMID 18270539. S2CID 4356708.
- Bininda-Emonds OR, Cardillo M, Jones KE, MacPhee RD, Beck RM, Grenyer R, et al. (March 2007). "The delayed rise of present-day mammals" (PDF). Nature. 446 (7135): 507–12. Bibcode:2007Natur.446..507B. doi:10.1038/nature05634. PMID 17392779. S2CID 4314965.
- Wible JR, Rougier GW, Novacek MJ, Asher RJ (June 2007). "Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary". Nature. 447 (7147): 1003–6. Bibcode:2007Natur.447.1003W. doi:10.1038/nature05854. PMID 17581585. S2CID 4334424.
- O'Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, et al. (February 2013). "The placental mammal ancestor and the post-K-Pg radiation of placentals". Science. 339 (6120): 662–7. Bibcode:2013Sci...339..662O. doi:10.1126/science.1229237. PMID 23393258. S2CID 206544776.
- Halliday TJ, Upchurch P, Goswami A (February 2017). "Resolving the relationships of Paleocene placental mammals". Biological Reviews of the Cambridge Philosophical Society. 92 (1): 521–550. doi:10.1111/brv.12242. PMC 6849585. PMID 28075073.
- Halliday TJ, Upchurch P, Goswami A (June 2016). "Eutherians experienced elevated evolutionary rates in the immediate aftermath of the Cretaceous-Palaeogene mass extinction". Proceedings. Biological Sciences. 283 (1833): 20153026. doi:10.1098/rspb.2015.3026. PMC 4936024. PMID 27358361.
- Ni X, Gebo DL, Dagosto M, Meng J, Tafforeau P, Flynn JJ, Beard KC (June 2013). "The oldest known primate skeleton and early haplorhine evolution". Nature. 498 (7452): 60–4. Bibcode:2013Natur.498...60N. doi:10.1038/nature12200. PMID 23739424. S2CID 4321956.
- Romer SA, Parsons TS (1977). The Vertebrate Body. Philadelphia: Holt-Saunders International. pp. 129–145. ISBN 978-0-03-910284-5. OCLC 60007175.
- Purves WK, Sadava DE, Orians GH, Helle HC (2001). Life: The Science of Biology (6 ed.). New York: Sinauer Associates, Inc. p. 593. ISBN 978-0-7167-3873-2. OCLC 874883911.
- Anthwal N, Joshi L, Tucker AS (January 2013). "Evolution of the mammalian middle ear and jaw: adaptations and novel structures". Journal of Anatomy. 222 (1): 147–60. doi:10.1111/j.1469-7580.2012.01526.x. PMC 3552421. PMID 22686855.
- van Nievelt AF, Smith KK (2005). "To replace or not to replace: the significance of reduced functional tooth replacement in marsupial and placental mammals". Paleobiology. 31 (2): 324–346. doi:10.1666/0094-8373(2005)031[0324:trontr]2.0.co;2.
- Libertini, G.; Ferrara, N. (2016). "Aging of perennial cells and organ parts according to the programmed aging paradigm". AGE. 38 (35): 35. doi:10.1007/s11357-016-9895-0. PMC 5005898. PMID 26957493.
- Mao F, Wang Y, Meng J (2015). "A Systematic Study on Tooth Enamel Microstructures of Lambdopsalis bulla (Multituberculate, Mammalia)--Implications for Multituberculate Biology and Phylogeny". PLOS ONE. 10 (5): e0128243. Bibcode:2015PLoSO..1028243M. doi:10.1371/journal.pone.0128243. PMC 4447277. PMID 26020958.
- Osborn HF (1900). "Origin of the Mammalia, III. Occipital Condyles of Reptilian Tripartite Type". The American Naturalist. 34 (408): 943–947. doi:10.1086/277821. JSTOR 2453526.
- Crompton AW, Jenkins Jr FA (1973). "Mammals from Reptiles: A Review of Mammalian Origins". Annual Review of Earth and Planetary Sciences. 1: 131–155. Bibcode:1973AREPS...1..131C. doi:10.1146/annurev.ea.01.050173.001023.
- Power ML, Schulkin J (2013). The Evolution Of The Human Placenta. Baltimore: Johns Hopkins University Press. pp. 1890–1891. ISBN 978-1-4214-0643-5. OCLC 940749490.
- Lindenfors, Patrik; Gittleman, John L.; Jones, Kate (2007), "Sexual size dimorphism in mammals", Sex, size and gender roles, pp. , s. 16–26, ISBN 978-0-19-920878-4, retrieved 2020-10-08
- Dierauf LA, Gulland FM (2001). CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation (2 ed.). Boca Raton: CRC Press. p. 154. ISBN 978-1-4200-4163-7. OCLC 166505919.
- Lui JH, Hansen DV, Kriegstein AR (July 2011). "Development and evolution of the human neocortex". Cell. 146 (1): 18–36. doi:10.1016/j.cell.2011.06.030. PMC 3610574. PMID 21729779.
- Keeler CE (June 1933). "Absence of the Corpus Callosum as a Mendelizing Character in the House Mouse". Proceedings of the National Academy of Sciences of the United States of America. 19 (6): 609–11. Bibcode:1933PNAS...19..609K. doi:10.1073/pnas.19.6.609. JSTOR 86284. PMC 1086100. PMID 16587795.
- Levitzky MG (2013). "Mechanics of Breathing". Pulmonary physiology (8 ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-179313-1. OCLC 940633137.
- Umesh KB (2011). "Pulmonary Anatomy and Physiology". Handbook of Mechanical Ventilation (1 ed.). New Delhi: Jaypee Brothers Medical Publishing. p. 12. ISBN 978-93-80704-74-6. OCLC 945076700.
- Standring S, Borley NR (2008). Gray's anatomy: the anatomical basis of clinical practice (40 ed.). London: Churchill Livingstone. pp. 960–962. ISBN 978-0-8089-2371-8. OCLC 213447727.
- Betts JG, Desaix P, Johnson E, Johnson JE, Korol O, Kruse D, Poe B, Wise JA, Womble M, Young KA (2013). Anatomy & physiology. Houston: Rice University Press. pp. 787–846. ISBN 978-1-938168-13-0. OCLC 898069394.
- Feldhamer GA, Drickamer LC, Vessey SH, Merritt JH, Krajewski C (2007). Mammalogy: Adaptation, Diversity, Ecology (3 ed.). Baltimore: Johns Hopkins University Press. ISBN 978-0-8018-8695-9. OCLC 124031907.
- Tinker SW (1988). Whales of the World. Brill Archive. p. 51. ISBN 978-0-935848-47-2.
- Romer AS (1959). The vertebrate story (4 ed.). Chicago: University of Chicago Press. ISBN 978-0-226-72490-4.
- de Muizon C, Lange-Badré B (1997). "Carnivorous dental adaptations in tribosphenic mammals and phylogenetic reconstruction". Lethaia. 30 (4): 353–366. doi:10.1111/j.1502-3931.1997.tb00481.x.
- Langer P (July 1984). "Comparative anatomy of the stomach in mammalian herbivores". Quarterly Journal of Experimental Physiology. 69 (3): 615–25. doi:10.1113/expphysiol.1984.sp002848. PMID 6473699.
- Vaughan TA, Ryan JM, Czaplewski NJ (2011). "Perissodactyla". Mammalogy (5 ed.). Jones and Bartlett. p. 322. ISBN 978-0-7637-6299-5. OCLC 437300511.
- Flower WH, Lydekker R (1946). An Introduction to the Study of Mammals Living and Extinct. London: Adam and Charles Black. p. 496. ISBN 978-1-110-76857-8.
- Sreekumar S (2010). Basic Physiology. PHI Learning Pvt. Ltd. pp. 180–181. ISBN 978-81-203-4107-4.
- Cheifetz AS (2010). Oxford American Handbook of Gastroenterology and Hepatology. Oxford: Oxford University Press, US. p. 165. ISBN 978-0-19-983012-1.
- Kuntz E (2008). Hepatology: Textbook and Atlas. Germany: Springer. p. 38. ISBN 978-3-540-76838-8.
- Ortiz RM (June 2001). "Osmoregulation in marine mammals". The Journal of Experimental Biology. 204 (Pt 11): 1831–44. PMID 11441026.
- Roman AS, Parsons TS (1977). The Vertebrate Body. Philadelphia: Holt-Saunders International. pp. 396–399. ISBN 978-0-03-910284-5.
- Biological Reviews – Cambridge Journals
- Dawkins R, Wong Y (2016). The Ancestor's Tale: A Pilgrimage to the Dawn of Evolution (2nd ed.). Boston: Mariner Books. p. 281. ISBN 978-0-544-85993-7.
- Fitch WT (2006). "Production of Vocalizations in Mammals" (PDF). In Brown K (ed.). Encyclopedia of Language and Linguistics. Oxford: Elsevier. pp. 115–121.
- Langevin P, Barclay RM (1990). "Hypsignathus monstrosus". Mammalian Species. 357 (357): 1–4. doi:10.2307/3504110. JSTOR 3504110.
- Weissengruber GE, Forstenpointner G, Peters G, Kübber-Heiss A, Fitch WT (September 2002). "Hyoid apparatus and pharynx in the lion (Panthera leo), jaguar (Panthera onca), tiger (Panthera tigris), cheetah (Acinonyxjubatus) and domestic cat (Felis silvestris f. catus)". Journal of Anatomy. 201 (3): 195–209. doi:10.1046/j.1469-7580.2002.00088.x. PMC 1570911. PMID 12363272.
- Stoeger AS, Heilmann G, Zeppelzauer M, Ganswindt A, Hensman S, Charlton BD (2012). "Visualizing sound emission of elephant vocalizations: evidence for two rumble production types". PLOS ONE. 7 (11): e48907. Bibcode:2012PLoSO...748907S. doi:10.1371/journal.pone.0048907. PMC 3498347. PMID 23155427.
- Clark CW (2004). "Baleen whale infrasonic sounds: Natural variability and function". Journal of the Acoustical Society of America. 115 (5): 2554. Bibcode:2004ASAJ..115.2554C. doi:10.1121/1.4783845.
- Dawson TJ, Webster KN, Maloney SK (February 2014). "The fur of mammals in exposed environments; do crypsis and thermal needs necessarily conflict? The polar bear and marsupial koala compared". Journal of Comparative Physiology B. 184 (2): 273–84. doi:10.1007/s00360-013-0794-8. PMID 24366474. S2CID 9481486.
- Slominski A, Tobin DJ, Shibahara S, Wortsman J (October 2004). "Melanin pigmentation in mammalian skin and its hormonal regulation". Physiological Reviews. 84 (4): 1155–228. doi:10.1152/physrev.00044.2003. PMID 15383650. S2CID 21168932.
- Hilton Jr B (1996). "South Carolina Wildlife". Animal Colors. Hilton Pond Center. 43 (4): 10–15. Retrieved 26 November 2011.
- Prum RO, Torres RH (May 2004). "Structural colouration of mammalian skin: convergent evolution of coherently scattering dermal collagen arrays" (PDF). The Journal of Experimental Biology. 207 (Pt 12): 2157–72. doi:10.1242/jeb.00989. hdl:1808/1599. PMID 15143148. S2CID 8268610.
- Suutari M, Majaneva M, Fewer DP, Voirin B, Aiello A, Friedl T, et al. (March 2010). "Molecular evidence for a diverse green algal community growing in the hair of sloths and a specific association with Trichophilus welckeri (Chlorophyta, Ulvophyceae)". BMC Evolutionary Biology. 10 (86): 86. doi:10.1186/1471-2148-10-86. PMC 2858742. PMID 20353556.
- Caro T (2005). "The Adaptive Significance of Coloration in Mammals". BioScience. 55 (2): 125–136. doi:10.1641/0006-3568(2005)055[0125:tasoci]2.0.co;2.
- Mills LS, Zimova M, Oyler J, Running S, Abatzoglou JT, Lukacs PM (April 2013). "Camouflage mismatch in seasonal coat color due to decreased snow duration". Proceedings of the National Academy of Sciences of the United States of America. 110 (18): 7360–5. Bibcode:2013PNAS..110.7360M. doi:10.1073/pnas.1222724110. PMC 3645584. PMID 23589881.
- Caro T (February 2009). "Contrasting coloration in terrestrial mammals". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 364 (1516): 537–48. doi:10.1098/rstb.2008.0221. PMC 2674080. PMID 18990666.
- Plavcan JM (2001). "Sexual dimorphism in primate evolution". American Journal of Physical Anthropology. Suppl 33 (33): 25–53. doi:10.1002/ajpa.10011. PMID 11786990.
- Bradley BJ, Gerald MS, Widdig A, Mundy NI (2012). "Coat Color Variation and Pigmentation Gene Expression in Rhesus Macaques (Macaca Mulatta)" (PDF). Journal of Mammalian Evolution. 20 (3): 263–270. doi:10.1007/s10914-012-9212-3. S2CID 13916535. Archived from the original (PDF) on 2015-09-24.
- Caro T, Izzo A, Reiner RC, Walker H, Stankowich T (April 2014). "The function of zebra stripes". Nature Communications. 5: 3535. Bibcode:2014NatCo...5.3535C. doi:10.1038/ncomms4535. PMID 24691390. S2CID 9849814.
- Lombardi J (30 November 1998). Comparative Vertebrate Reproduction. Springer Science & Business Media. ISBN 978-0-7923-8336-9.
- Tyndale-Biscoe H, Renfree M (30 January 1987). Reproductive Physiology of Marsupials. Cambridge University Press. ISBN 978-0-521-33792-2.
- Johnston SD, Smith B, Pyne M, Stenzel D, Holt WV (2007). "One‐Sided Ejaculation of Echidna Sperm Bundles" (PDF). The American Naturalist. 170 (6): E162–E164. doi:10.1086/522847. PMID 18171162.
- Maxwell KE (2013). The Sex Imperative: An Evolutionary Tale of Sexual Survival. Springer. pp. 112–113. ISBN 978-1-4899-5988-1.
- Vaughan TA, Ryan JP, Czaplewski NJ (2011). Mammalogy. Jones & Bartlett Publishers. p. 387. ISBN 978-0-03-025034-7.
- Hoffman EA, Rowe TB (September 2018). "Jurassic stem-mammal perinates and the origin of mammalian reproduction and growth". Nature. 561 (7721): 104–108. Bibcode:2018Natur.561..104H. doi:10.1038/s41586-018-0441-3. PMID 30158701. S2CID 205570021.
- Wallis MC, Waters PD, Delbridge ML, Kirby PJ, Pask AJ, Grützner F, et al. (2007). "Sex determination in platypus and echidna: autosomal location of SOX3 confirms the absence of SRY from monotremes". Chromosome Research. 15 (8): 949–59. doi:10.1007/s10577-007-1185-3. PMID 18185981. S2CID 812974.
- Marshall Graves JA (2008). "Weird animal genomes and the evolution of vertebrate sex and sex chromosomes" (PDF). Annual Review of Genetics. 42: 565–86. doi:10.1146/annurev.genet.42.110807.091714. PMID 18983263. Archived from the original (PDF) on 2012-09-04.
- Novacek MJ, Rougier GW, Wible JR, McKenna MC, Dashzeveg D, Horovitz I (October 1997). "Epipubic bones in eutherian mammals from the late Cretaceous of Mongolia". Nature. 389 (6650): 483–6. Bibcode:1997Natur.389..483N. doi:10.1038/39020. PMID 9333234. S2CID 205026882.
- Morgan S (2005). "Mammal Behavior and Lifestyle". Mammals. Chicago: Raintree. p. 6. ISBN 978-1-4109-1050-9. OCLC 53476660.
- Verma PS, Pandey BP (2013). ISC Biology Book I for Class XI. New Delhi: S. Chand and Company. p. 288. ISBN 978-81-219-2557-0.
- Oftedal OT (July 2002). "The mammary gland and its origin during synapsid evolution". Journal of Mammary Gland Biology and Neoplasia. 7 (3): 225–52. doi:10.1023/a:1022896515287. PMID 12751889. S2CID 25806501.
- Krockenberger A (2006). "Lactation". In Dickman CR, Armati PJ, Hume ID (eds.). Marsupials. p. 109. ISBN 978-1-139-45742-2.
- Schulkin J, Power ML (2016). Milk: The Biology of Lactation. Johns Hopkins University Press. p. 66. ISBN 978-1-4214-2042-4.
- Thompson KV, Baker AJ, Baker AM (2010). "Paternal Care and Behavioral Development in Captive Mammals". In Baer CK, Kleiman DG, Thompson KV (eds.). Wild Mammals in Captivity Principles and Techniques for Zoo Management (2nd ed.). University of Chicago Press. p. 374. ISBN 978-0-226-44011-8.
- Campbell NA, Reece JB (2002). Biology (6 ed.). Benjamin Cummings. p. 845. ISBN 978-0-8053-6624-2. OCLC 47521441.
- Buffenstein R, Yahav S (1991). "Is the naked mole-rat Hererocephalus glaber an endothermic yet poikilothermic mammal?". Journal of Thermal Biology. 16 (4): 227–232. doi:10.1016/0306-4565(91)90030-6.
- Schmidt-Nielsen K, Duke JB (1997). "Temperature Effects". Animal Physiology: Adaptation and Environment (5 ed.). Cambridge. p. 218. ISBN 978-0-521-57098-5. OCLC 35744403.
- Lorenzini A, Johnson FB, Oliver A, Tresini M, Smith JS, Hdeib M, et al. (2009). "Significant correlation of species longevity with DNA double strand break recognition but not with telomere length". Mechanisms of Ageing and Development. 130 (11–12): 784–92. doi:10.1016/j.mad.2009.10.004. PMC 2799038. PMID 19896964.
- Hart RW, Setlow RB (June 1974). "Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species". Proceedings of the National Academy of Sciences of the United States of America. 71 (6): 2169–73. Bibcode:1974PNAS...71.2169H. doi:10.1073/pnas.71.6.2169. PMC 388412. PMID 4526202.
- Ma S, Upneja A, Galecki A, Tsai YM, Burant CF, Raskind S, et al. (November 2016). "Cell culture-based profiling across mammals reveals DNA repair and metabolism as determinants of species longevity". eLife. 5. doi:10.7554/eLife.19130. PMC 5148604. PMID 27874830.
- Grube K, Bürkle A (December 1992). "Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span". Proceedings of the National Academy of Sciences of the United States of America. 89 (24): 11759–63. Bibcode:1992PNAS...8911759G. doi:10.1073/pnas.89.24.11759. PMC 50636. PMID 1465394.
- Francis AA, Lee WH, Regan JD (June 1981). "The relationship of DNA excision repair of ultraviolet-induced lesions to the maximum life span of mammals". Mechanisms of Ageing and Development. 16 (2): 181–9. doi:10.1016/0047-6374(81)90094-4. PMID 7266079. S2CID 19830165.
- Treton JA, Courtois Y (March 1982). "Correlation between DNA excision repair and mammalian lifespan in lens epithelial cells". Cell Biology International Reports. 6 (3): 253–60. doi:10.1016/0309-1651(82)90077-7. PMID 7060140.
- Maslansky CJ, Williams GM (February 1985). "Ultraviolet light-induced DNA repair synthesis in hepatocytes from species of differing longevities". Mechanisms of Ageing and Development. 29 (2): 191–203. doi:10.1016/0047-6374(85)90018-1. PMID 3974310. S2CID 23988416.
- "Leg and feet". Avian Skeletal Adaptations. Archived from the original on 2008-04-04. Retrieved 3 August 2008.
- Walker WF, Homberger DG (1998). Anatomy and Dissection of the Fetal Pig (5 ed.). New York: W. H. Freeman and Company. p. 3. ISBN 978-0-7167-2637-1. OCLC 40576267.
- Orr CM (November 2005). "Knuckle-walking anteater: a convergence test of adaptation for purported knuckle-walking features of African Hominidae". American Journal of Physical Anthropology. 128 (3): 639–58. doi:10.1002/ajpa.20192. PMID 15861420.
- Fish FE, Frappell PB, Baudinette RV, MacFarlane PM (February 2001). "Energetics of terrestrial locomotion of the platypus Ornithorhynchus anatinus" (PDF). The Journal of Experimental Biology. 204 (Pt 4): 797–803. PMID 11171362.
- Dhingra P (2004). "Comparative Bipedalism – How the Rest of the Animal Kingdom Walks on two legs". Anthropological Science. 131 (231).
- Alexander RM (May 2004). "Bipedal animals, and their differences from humans". Journal of Anatomy. 204 (5): 321–30. doi:10.1111/j.0021-8782.2004.00289.x. PMC 1571302. PMID 15198697.
- Dagg AI (1973). "Gaits in Mammals". Mammal Review. 3 (4): 135–154. doi:10.1111/j.1365-2907.1973.tb00179.x.
- Roberts TD (1995). Understanding Balance: The Mechanics of Posture and Locomotion. San Diego: Nelson Thornes. p. 211. ISBN 978-1-56593-416-0. OCLC 33167785.
- Cartmill M (1985). "Climbing". In Hildebrand M, Bramble DM, Liem KF, Wake DB (eds.). Functional Vertebrate Morphology. Cambridge: Belknap Press. pp. 73–88. ISBN 978-0-674-32775-7. OCLC 11114191.
- Vernes K (2001). "Gliding Performance of the Northern Flying Squirrel (Glaucomys sabrinus) in Mature Mixed Forest of Eastern Canada" (PDF). Journal of Mammalogy. 82 (4): 1026–1033. doi:10.1644/1545-1542(2001)082<1026:GPOTNF>2.0.CO;2.
- Barba LA (October 2011). "Bats – the only flying mammals". Bio-Aerial Locomotion. Retrieved 20 May 2016.
- "Bats In Flight Reveal Unexpected Aerodynamics". ScienceDaily. 2007. Retrieved July 12, 2016.
- Hedenström A, Johansson LC (March 2015). "Bat flight: aerodynamics, kinematics and flight morphology" (PDF). The Journal of Experimental Biology. 218 (Pt 5): 653–63. doi:10.1242/jeb.031203. PMID 25740899. S2CID 21295393.
- "Bats save energy by drawing in wings on upstroke". ScienceDaily. 2012. Retrieved July 12, 2016.
- Taschek K (2008). Hanging with Bats: Ecobats, Vampires, and Movie Stars. Albuquerque: University of New Mexico Press. p. 14. ISBN 978-0-8263-4403-8. OCLC 191258477.
- Sterbing-D'Angelo S, Chadha M, Chiu C, Falk B, Xian W, Barcelo J, et al. (July 2011). "Bat wing sensors support flight control". Proceedings of the National Academy of Sciences of the United States of America. 108 (27): 11291–6. Bibcode:2011PNAS..10811291S. doi:10.1073/pnas.1018740108. PMC 3131348. PMID 21690408.
- Damiani, R, 2003, Earliest evidence of cynodont burrowing, The Royal Society Publishing, Volume 270, Issue 1525
- Shimer HW (1903). "Adaptations to Aquatic, Arboreal, Fossorial and Cursorial Habits in Mammals. III. Fossorial Adaptations". The American Naturalist. 37 (444): 819–825. doi:10.1086/278368. JSTOR 2455381.
- Stanhope, M. J.; Waddell, V. G.; et al. (1998). "Molecular evidence for multiple origins of Insectivora and for a new order of endemic African insectivore mammals". Proceedings of the National Academy of Sciences. 95 (17): 9967–9972. Bibcode:1998PNAS...95.9967S. doi:10.1073/pnas.95.17.9967. PMC 21445. PMID 9707584.
- Perry DA (1949). "The anatomical basis of swimming in Whales". Journal of Zoology. 119 (1): 49–60. doi:10.1111/j.1096-3642.1949.tb00866.x.
- Fish FE, Hui CA (1991). "Dolphin swimming – a review" (PDF). Mammal Review. 21 (4): 181–195. doi:10.1111/j.1365-2907.1991.tb00292.x. Archived from the original (PDF) on 2006-08-29.
- Marsh H (1989). "Chapter 57: Dugongidae" (PDF). Fauna of Australia. 1. Canberra: Australian Government Publications. ISBN 978-0-644-06056-1. OCLC 27492815. Archived from the original on 2013-05-11.CS1 maint: bot: original URL status unknown (link)
- Berta A (April 2012). "Pinniped Diversity: Evolution and Adaptations". Return to the Sea: The Life and Evolutionary Times of Marine Mammals. University of California Press. pp. 62–64. ISBN 978-0-520-27057-2.
- Fish FE, Hurley J, Costa DP (February 2003). "Maneuverability by the sea lion Zalophus californianus: turning performance of an unstable body design". The Journal of Experimental Biology. 206 (Pt 4): 667–74. doi:10.1242/jeb.00144. PMID 12517984.
- Riedman M (1990). The Pinnipeds: Seals, Sea Lions, and Walruses. University of California Press. ISBN 978-0-520-06497-3. OCLC 19511610.
- Fish FE (1996). "Transitions from drag-based to lift-based propulsion in mammalian swimming". Integrative and Comparative Biology. 36 (6): 628–641. doi:10.1093/icb/36.6.628.
- Fish FE (2000). "Biomechanics and energetics in aquatic and semiaquatic mammals: platypus to whale" (PDF). Physiological and Biochemical Zoology. 73 (6): 683–98. CiteSeerX 10.1.1.734.1217. doi:10.1086/318108. PMID 11121343. Archived from the original (PDF) on 2016-08-04.
- Eltringham SK (1999). "Anatomy and Physiology". The Hippos. London: T & AD Poyser Ltd. p. 8. ISBN 978-0-85661-131-5. OCLC 42274422.
- "Hippopotamus Hippopotamus amphibius". National Geographic. Archived from the original on 2014-11-25. Retrieved 30 April 2016.
- Seyfarth RM, Cheney DL, Marler P (1980). "Vervet Monkey Alarm Calls: Semantic communication in a Free-Ranging Primate". Animal Behaviour. 28 (4): 1070–1094. doi:10.1016/S0003-3472(80)80097-2. S2CID 53165940.
- Zuberbühler K (2001). "Predator-specific alarm calls in Campbell's monkeys, Cercopithecus campbelli". Behavioral Ecology and Sociobiology. 50 (5): 414–442. doi:10.1007/s002650100383. JSTOR 4601985. S2CID 21374702.
- Slabbekoorn H, Smith TB (April 2002). "Bird song, ecology and speciation". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 357 (1420): 493–503. doi:10.1098/rstb.2001.1056. PMC 1692962. PMID 12028787.
- Bannister JL (2008). "Baleen Whales (Mysticetes)". In F Perrin W, Würsig B, Thewissen JG (eds.). Encyclopedia of Marine Mammals (2 ed.). Academic Press. pp. 80–89. ISBN 978-0-12-373553-9.
- Norris S (2002). "Creatures of Culture? Making the Case for Cultural Systems in Whales and Dolphins" (PDF). BioScience. 52 (1): 9–14. doi:10.1641/0006-3568(2002)052[0009:COCMTC]2.0.CO;2.
- Boughman JW (February 1998). "Vocal learning by greater spear-nosed bats". Proceedings. Biological Sciences. 265 (1392): 227–33. doi:10.1098/rspb.1998.0286. PMC 1688873. PMID 9493408.
- "Prairie dogs' language decoded by scientists". CBC News. 21 June 2013. Retrieved 20 May 2015.
- Mayell H (3 March 2004). "Elephants Call Long-Distance After-Hours". National Geographic. Retrieved 15 November 2016.
- Maynard Smith J, Harper D (2003). Animal Signals. Oxford Series in Ecology and Evolution. Oxford University Press. pp. 61–63. ISBN 978-0-19-852684-1. OCLC 54460090.
- FitzGibbon CD, Fanshawe JH (1988). "Stotting in Thomson's gazelles: an honest signal of condition" (PDF). Behavioral Ecology and Sociobiology. 23 (2): 69–74. doi:10.1007/bf00299889. S2CID 2809268. Archived from the original (PDF) on 2014-02-25.
- Bildstein KL (May 1983). "Why White-Tailed Deer Flag Their Tails". The American Naturalist. 121 (5): 709–715. doi:10.1086/284096. JSTOR 2460873.
- Gosling LM (January 1982). "A reassessment of the function of scent marking in territories". Zeitschrift für Tierpsychologie. 60 (2): 89–118. doi:10.1111/j.1439-0310.1982.tb00492.x.
- Zala SM, Potts WK, Penn DJ (March 2004). "Scent-marking displays provide honest signals of health and infection". Behavioral Ecology. 15 (2): 338–44. doi:10.1093/beheco/arh022. hdl:10.1093/beheco/arh022.
- Johnson RP (August 1973). "Scent Marking in Mammals". Animal Behaviour. 21 (3): 521–535. doi:10.1016/S0003-3472(73)80012-0.
- Schevill WE, McBride AF (1956). "Evidence for echolocation by cetaceans". Deep-Sea Research. 3 (2): 153–154. Bibcode:1956DSR.....3..153S. doi:10.1016/0146-6313(56)90096-x.
- Wilson W, Moss C (2004). Thomas J (ed.). Echolocation in Bats and Dolphins. Chicago University Press. p. 22. ISBN 978-0-226-79599-7. OCLC 50143737.
- Au WW (1993). The Sonar of Dolphins. Springer-Verlag. ISBN 978-3-540-97835-0. OCLC 26158593.
- Sanders JG, Beichman AC, Roman J, Scott JJ, Emerson D, McCarthy JJ, Girguis PR (September 2015). "Baleen whales host a unique gut microbiome with similarities to both carnivores and herbivores". Nature Communications. 6: 8285. Bibcode:2015NatCo...6.8285S. doi:10.1038/ncomms9285. PMC 4595633. PMID 26393325.
- Speaksman JR (1996). "Energetics and the evolution of body size in small terrestrial mammals" (PDF). Symposia of the Zoological Society of London (69): 69–81.
- Wilson DE, Burnie D, eds. (2001). Animal: The Definitive Visual Guide to the World's Wildlife (1st ed.). DK Publishing. pp. 86–89. ISBN 978-0-7894-7764-4. OCLC 46422124.
- Van Valkenburgh B (July 2007). "Deja vu: the evolution of feeding morphologies in the Carnivora". Integrative and Comparative Biology. 47 (1): 147–63. doi:10.1093/icb/icm016. PMID 21672827.
- Sacco T, van Valkenburgh B (2004). "Ecomorphological indicators of feeding behaviour in the bears (Carnivora: Ursidae)". Journal of Zoology. 263 (1): 41–54. doi:10.1017/S0952836904004856.
- Singer MS, Bernays EA (2003). "Understanding omnivory needs a behavioral perspective". Ecology. 84 (10): 2532–2537. doi:10.1890/02-0397.
- Hutson JM, Burke CC, Haynes G (2013-12-01). "Osteophagia and bone modifications by giraffe and other large ungulates". Journal of Archaeological Science. 40 (12): 4139–4149. doi:10.1016/j.jas.2013.06.004.
- "Why Do Cats Eat Grass?". Pet MD. Retrieved 13 January 2017.
- Geiser F (2004). "Metabolic rate and body temperature reduction during hibernation and daily torpor". Annual Review of Physiology. 66: 239–74. doi:10.1146/annurev.physiol.66.032102.115105. PMID 14977403. S2CID 22397415.
- Humphries MM, Thomas DW, Kramer DL (2003). "The role of energy availability in Mammalian hibernation: a cost-benefit approach". Physiological and Biochemical Zoology. 76 (2): 165–79. doi:10.1086/367950. PMID 12794670. S2CID 14675451.
- Barnes BM (June 1989). "Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator". Science. 244 (4912): 1593–5. Bibcode:1989Sci...244.1593B. doi:10.1126/science.2740905. PMID 2740905.
- Geiser F (2010). "Aestivation in Mammals and Birds". In Navas CA, Carvalho JE (eds.). Aestivation: Molecular and Physiological Aspects. Progress in Molecular and Subcellular Biology. 49. Springer-Verlag. pp. 95–113. doi:10.1007/978-3-642-02421-4. ISBN 978-3-642-02420-7.
- Mann J, Patterson EM (November 2013). "Tool use by aquatic animals". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 368 (1630): 20120424. doi:10.1098/rstb.2012.0424. PMC 4027413. PMID 24101631.
- Raffaele P (2011). Among the Great Apes: Adventures on the Trail of Our Closest Relatives. New York: Harper. p. 83. ISBN 978-0-06-167184-5. OCLC 674694369.
- Köhler W (1925). The Mentality of Apes. Liveright. ISBN 978-0-87140-108-3. OCLC 2000769.
- McGowan RT, Rehn T, Norling Y, Keeling LJ (May 2014). "Positive affect and learning: exploring the "Eureka Effect" in dogs". Animal Cognition. 17 (3): 577–87. doi:10.1007/s10071-013-0688-x. PMID 24096703. S2CID 15216926.
- Karbowski J (May 2007). "Global and regional brain metabolic scaling and its functional consequences". BMC Biology. 5 (18): 18. arXiv:0705.2913. Bibcode:2007arXiv0705.2913K. doi:10.1186/1741-7007-5-18. PMC 1884139. PMID 17488526.
- Marino L (June 2007). "Cetacean brains: how aquatic are they?". Anatomical Record. 290 (6): 694–700. doi:10.1002/ar.20530. PMID 17516433.
- Gallop GG (January 1970). "Chimpanzees: self-recognition". Science. 167 (3914): 86–7. Bibcode:1970Sci...167...86G. doi:10.1126/science.167.3914.86. PMID 4982211. S2CID 145295899.
- Plotnik JM, de Waal FB, Reiss D (November 2006). "Self-recognition in an Asian elephant" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 103 (45): 17053–7. Bibcode:2006PNAS..10317053P. doi:10.1073/pnas.0608062103. PMC 1636577. PMID 17075063.
- Robert S (1986). "Ontogeny of mirror behavior in two species of great apes". American Journal of Primatology. 10 (2): 109–117. doi:10.1002/ajp.1350100202. PMID 31979488.
- Walraven V, van Elsacker L, Verheyen R (1995). "Reactions of a group of pygmy chimpanzees (Pan paniscus) to their mirror images: evidence of self-recognition". Primates. 36: 145–150. doi:10.1007/bf02381922. S2CID 38985498.
- Leakey R (1994). "The Origin of the Mind". The Origin Of Humankind. New York: BasicBooks. p. 150. ISBN 978-0-465-05313-1. OCLC 30739453.
- Archer J (1992). Ethology and Human Development. Rowman & Littlefield. pp. 215–218. ISBN 978-0-389-20996-6. OCLC 25874476.
- Marten K, Psarakos S (1995). "Evidence of self-awareness in the bottlenose dolphin (Tursiops truncatus)". In Parker ST, Mitchell R, Boccia M (eds.). Self-awareness in Animals and Humans: Developmental Perspectives. Cambridge: Cambridge University Press. pp. 361–379. ISBN 978-0-521-44108-7. OCLC 28180680.
- Delfour F, Marten K (April 2001). "Mirror image processing in three marine mammal species: killer whales (Orcinus orca), false killer whales (Pseudorca crassidens) and California sea lions (Zalophus californianus)". Behavioural Processes. 53 (3): 181–190. doi:10.1016/s0376-6357(01)00134-6. PMID 11334706. S2CID 31124804.
- Jarvis JU (May 1981). "Eusociality in a mammal: cooperative breeding in naked mole-rat colonies". Science. 212 (4494): 571–3. Bibcode:1981Sci...212..571J. doi:10.1126/science.7209555. JSTOR 1686202. PMID 7209555. S2CID 880054.
- Jacobs DS, et al. (1991). "The colony structure and dominance hierarchy of the Damaraland mole-rat, Cryptomys damarensis (Rodentia: Bathyergidae) from Namibia". Journal of Zoology. 224 (4): 553–576. doi:10.1111/j.1469-7998.1991.tb03785.x.
- Hardy SB (2009). Mothers and Others: The Evolutionary Origins of Mutual Understanding. Boston: Belknap Press of Harvard University Press. pp. 92–93.
- Harlow HF, Suomi SJ (July 1971). "Social recovery by isolation-reared monkeys". Proceedings of the National Academy of Sciences of the United States of America. 68 (7): 1534–8. Bibcode:1971PNAS...68.1534H. doi:10.1073/pnas.68.7.1534. PMC 389234. PMID 5283943.
- van Schaik CP (January 1999). "The socioecology of fission-fusion sociality in Orangutans". Primates; Journal of Primatology. 40 (1): 69–86. doi:10.1007/BF02557703. PMID 23179533. S2CID 13366732.
- Archie EA, Moss CJ, Alberts SC (March 2006). "The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants". Proceedings. Biological Sciences. 273 (1586): 513–22. doi:10.1098/rspb.2005.3361. PMC 1560064. PMID 16537121.
- Smith JE, Memenis SK, Holekamp KE (2007). "Rank-related partner choice in the fission–fusion society of the spotted hyena (Crocuta crocuta)" (PDF). Behavioral Ecology and Sociobiology. 61 (5): 753–765. doi:10.1007/s00265-006-0305-y. S2CID 24927919. Archived from the original (PDF) on 2014-04-25.
- Matoba T, Kutsukake N, Hasegawa T (2013). Hayward M (ed.). "Head rubbing and licking reinforce social bonds in a group of captive African lions, Panthera leo". PLOS ONE. 8 (9): e73044. Bibcode:2013PLoSO...873044M. doi:10.1371/journal.pone.0073044. PMC 3762833. PMID 24023806.
- Krützen M, Barré LM, Connor RC, Mann J, Sherwin WB (July 2004). "'O father: where art thou?'--Paternity assessment in an open fission-fusion society of wild bottlenose dolphins (Tursiops sp.) in Shark Bay, Western Australia". Molecular Ecology. 13 (7): 1975–90. doi:10.1111/j.1365-294X.2004.02192.x. PMID 15189218. S2CID 4510393.
- Martin C (1991). The Rainforests of West Africa: Ecology – Threats – Conservation (1 ed.). Springer. doi:10.1007/978-3-0348-7726-8. ISBN 978-3-0348-7726-8.
- le Roux A, Cherry MI, Gygax L (5 May 2009). "Vigilance behaviour and fitness consequences: comparing a solitary foraging and an obligate group-foraging mammal". Behavioral Ecology and Sociobiology. 63 (8): 1097–1107. doi:10.1007/s00265-009-0762-1. S2CID 21961356.
- Palagi E, Norscia I (2015). Samonds KE (ed.). "The Season for Peace: Reconciliation in a Despotic Species (Lemur catta)". PLOS ONE. 10 (11): e0142150. Bibcode:2015PLoSO..1042150P. doi:10.1371/journal.pone.0142150. PMC 4646466. PMID 26569400.
- East ML, Hofer H (2000). "Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females". Behavioral Ecology. 12 (15): 558–568. doi:10.1093/beheco/12.5.558.
- Samuels A, Silk JB, Rodman P (1984). "Changes in the dominance rank and reproductive behavior of male bonnet macaques (Macaca radiate)". Animal Behaviour. 32 (4): 994–1003. doi:10.1016/s0003-3472(84)80212-2. S2CID 53186523.
- Delpietro HA, Russo RG (2002). "Observations of the common vampire bat (Desmodus rotundus) and the hairy-legged vampire bat (Diphylla ecaudata) in captivity". Mammalian Biology. 67 (2): 65–78. doi:10.1078/1616-5047-00011.
- Kleiman DG (March 1977). "Monogamy in mammals". The Quarterly Review of Biology. 52 (1): 39–69. doi:10.1086/409721. PMID 857268. S2CID 25675086.
- Holland B, Rice WR (February 1998). "Perspective: Chase-Away Sexual Selection: Antagonistic Seduction Versus Resistance" (PDF). Evolution; International Journal of Organic Evolution. 52 (1): 1–7. doi:10.2307/2410914. JSTOR 2410914. PMID 28568154. Archived from the original (PDF) on 2019-06-08. Retrieved 2016-07-08.
- Clutton-Brock TH (May 1989). "Mammalian mating systems". Proceedings of the Royal Society of London. Series B, Biological Sciences. 236 (1285): 339–72. Bibcode:1989RSPSB.236..339C. doi:10.1098/rspb.1989.0027. PMID 2567517. S2CID 84780662.
- Boness DJ, Bowen D, Buhleier BM, Marshall GJ (2006). "Mating tactics and mating system of an aquatic-mating pinniped: the harbor seal, Phoca vitulina". Behavioral Ecology and Sociobiology. 61: 119–130. doi:10.1007/s00265-006-0242-9. S2CID 25266746.
- Klopfer PH (1981). "Origins of Parental Care". In Gubernick DJ (ed.). Parental Care in Mammals. New York: Plenum Press. ISBN 978-1-4613-3150-6. OCLC 913709574.
- Murthy R, Bearman G, Brown S, Bryant K, Chinn R, Hewlett A, et al. (May 2015). "Animals in healthcare facilities: recommendations to minimize potential risks" (PDF). Infection Control and Hospital Epidemiology. 36 (5): 495–516. doi:10.1017/ice.2015.15. PMID 25998315.
- The Humane Society of the United States. "U.S. Pet Ownership Statistics". Retrieved 27 April 2012.
- USDA. "U.S. Rabbit Industry profile" (PDF). Archived from the original (PDF) on 7 August 2019. Retrieved 10 July 2013.
- McKie R (26 May 2013). "Prehistoric cave art in the Dordogne". The Guardian. Retrieved 9 November 2016.
- Jones J (27 June 2014). "The top 10 animal portraits in art". The Guardian. Retrieved 24 June 2016.
- "Deer Hunting in the United States: An Analysis of Hunter Demographics and Behavior Addendum to the 2001 National Survey of Fishing, Hunting, and Wildlife-Associated Recreation Report 2001-6". Fishery and Wildlife Service (USA). Retrieved 24 June 2016.
- Shelton L (2014-04-05). "Recreational Hog Hunting Popularity Soaring". The Natchez Democrat. Gramd View Outdoors. Archived from the original on 12 December 2017. Retrieved 24 June 2016.
- Nguyen J, Wheatley R (2015). Hunting For Food: Guide to Harvesting, Field Dressing and Cooking Wild Game. F+W Media. pp. 6–77. ISBN 978-1-4403-3856-4. Chapters on hunting deer, wild hog (boar), rabbit, and squirrel.
- "Horse racing". The Encyclopædia Britannica. Archived from the original on 21 December 2013. Retrieved 6 May 2014.
- Genders R (1981). Encyclopaedia of Greyhound Racing. Pelham Books. ISBN 978-0-7207-1106-6. OCLC 9324926.
- Plous S (1993). "The Role of Animals in Human Society". Journal of Social Issues. 49 (1): 1–9. doi:10.1111/j.1540-4560.1993.tb00906.x.
- Fowler KJ (26 March 2014). "Top 10 books about intelligent animals". The Guardian. Retrieved 9 November 2016.
- Gamble N, Yates S (2008). Exploring Children's Literature (2 ed.). Los Angeles: Sage. ISBN 978-1-4129-3013-0. OCLC 71285210.
- "Books for Adults". Seal Sitters. Retrieved 9 November 2016.
- Paterson J (2013). "Animals in Film and Media". Oxford Bibliographies. doi:10.1093/obo/9780199791286-0044.
- Johns C (2011). Cattle: History, Myth, Art. London: The British Museum Press. ISBN 978-0-7141-5084-0. OCLC 665137673.
- van Gulik RH. Hayagrīva: The Mantrayānic Aspect of Horse-cult in China and Japan. Brill Archive. p. 9.
- Grainger R (24 June 2012). "Lion Depiction across Ancient and Modern Religions". ALERT. Archived from the original on 23 September 2016. Retrieved November 6, 2016.
- "Graphic detail Charts, maps and infographics. Counting chickens". The Economist. 27 July 2011. Retrieved 6 November 2016.
- "Breeds of Cattle at CATTLE TODAY". Cattle Today. Cattle-today.com. Retrieved November 6, 2016.
- Lukefahr SD, Cheeke PR. "Rabbit project development strategies in subsistence farming systems". Food and Agriculture Organization. Retrieved November 6, 2016.
- Pond WG (2004). Encyclopedia of Animal Science. CRC Press. pp. 248–250. ISBN 978-0-8247-5496-9. OCLC 57033325.
- "History of Leather". Moore & Giles. Retrieved 10 November 2016.
- Braaten AW (2005). "Wool". In Steele V (ed.). Encyclopedia of Clothing and Fashion. 3. Thomson Gale. pp. 441–443. ISBN 978-0-684-31394-8. OCLC 963977000.
- Quiggle C (Fall 2000). "Alpaca: An Ancient Luxury". Interweave Knits: 74–76.
- "Genetics Research". Animal Health Trust. Archived from the original on December 12, 2017. Retrieved November 6, 2016.
- "Drug Development". Animal Research.info. Retrieved November 6, 2016.
- "EU statistics show decline in animal research numbers". Speaking of Research. 2013. Retrieved November 6, 2016.
- Pilcher HR (2003). "It's a knockout". Nature. doi:10.1038/news030512-17. Retrieved November 6, 2016.
- "The supply and use of primates in the EU". European Biomedical Research Association. 1996. Archived from the original on 2012-01-17.
- Carlsson HE, Schapiro SJ, Farah I, Hau J (August 2004). "Use of primates in research: a global overview". American Journal of Primatology. 63 (4): 225–37. doi:10.1002/ajp.20054. PMID 15300710.
- Weatherall D, et al. (2006). The use of non-human primates in research (PDF) (Report). London, UK: Academy of Medical Sciences. Archived from the original (PDF) on 2013-03-23.
- Diamond JM (1997). "Part 2: The rise and spread of food production". Guns, Germs, and Steel: the Fates of Human Societies (1 ed.). New York: W.W. Norton & Company. ISBN 978-0-393-03891-0. OCLC 35792200.
- Larson G, Burger J (April 2013). "A population genetics view of animal domestication" (PDF). Trends in Genetics. 29 (4): 197–205. doi:10.1016/j.tig.2013.01.003. PMID 23415592.
- Zeder MA (August 2008). "Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact". Proceedings of the National Academy of Sciences of the United States of America. 105 (33): 11597–604. Bibcode:2008PNAS..10511597Z. doi:10.1073/pnas.0801317105. PMC 2575338. PMID 18697943.
- Price E (2008). Principles and applications of domestic animal behavior: an introductory text. Sacramento: Cambridge University Press. ISBN 978-1-84593-398-2. OCLC 226038028.
- Taupitz J, Weschka M (2009). Chimbrids – Chimeras and Hybrids in Comparative European and International Research. Heidelberg: Springer. p. 13. ISBN 978-3-540-93869-9. OCLC 495479133.
- Chambers SM, Fain SR, Fazio B, Amaral M (2012). "An account of the taxonomy of North American wolves from morphological and genetic analyses". North American Fauna. 77: 2. doi:10.3996/nafa.77.0001.
- van Vuure T (2005). Retracing the Aurochs – History, Morphology and Ecology of an extinct wild Ox. Pensoft Publishers. ISBN 978-954-642-235-4. OCLC 940879282.
- Mooney HA, Cleland EE (May 2001). "The evolutionary impact of invasive species". Proceedings of the National Academy of Sciences of the United States of America. 98 (10): 5446–51. Bibcode:2001PNAS...98.5446M. doi:10.1073/pnas.091093398. PMC 33232. PMID 11344292.
- Le Roux JJ, Foxcroft LC, Herbst M, MacFadyen S (January 2015). "Genetic analysis shows low levels of hybridization between African wildcats (Felis silvestris lybica) and domestic cats (F. s. catus) in South Africa". Ecology and Evolution. 5 (2): 288–99. doi:10.1002/ece3.1275. PMC 4314262. PMID 25691958.
- Wilson A (2003). Australia's state of the forests report. p. 107.
- Rhymer JM, Simberloff D (November 1996). "Extinction by Hybridization and Introgression". Annual Review of Ecology and Systematics. 27: 83–109. doi:10.1146/annurev.ecolsys.27.1.83.
- Potts BM (2001). Barbour RC, Hingston AB (eds.). Genetic pollution from farm forestry using eucalypt species and hybrids : a report for the RIRDC/L&WA/FWPRDC Joint Venture Agroforestry Program. Rural Industrial Research and Development Corporation of Australia. ISBN 978-0-642-58336-9. OCLC 48794104.
- Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B (July 2014). "Defaunation in the Anthropocene" (PDF). Science. 345 (6195): 401–6. Bibcode:2014Sci...345..401D. doi:10.1126/science.1251817. PMID 25061202. S2CID 206555761.
- Primack R (2014). Essentials of Conservation Biology (6 ed.). Sunderland, MA: Sinauer Associates, Inc. Publishers. pp. 217–245. ISBN 978-1-60535-289-3. OCLC 876140621.
- Vignieri S (July 2014). "Vanishing fauna. Introduction". Science. 345 (6195): 392–5. Bibcode:2014Sci...345..392V. doi:10.1126/science.345.6195.392. PMID 25061199.
- Burney DA, Flannery TF (July 2005). "Fifty millennia of catastrophic extinctions after human contact" (PDF). Trends in Ecology & Evolution. 20 (7): 395–401. doi:10.1016/j.tree.2005.04.022. PMID 16701402. Archived from the original on 2010-06-10.CS1 maint: bot: original URL status unknown (link)
- Diamond J (1984). "Historic extinctions: a Rosetta stone for understanding prehistoric extinctions". In Martin PS, Klein RG (eds.). Quaternary extinctions: A prehistoric revolution. Tucson: University of Arizona Press. pp. 824–862. ISBN 978-0-8165-1100-6. OCLC 10301944.
- Watts J (May 6, 2019). "Human society under urgent threat from loss of Earth's natural life". The Guardian. Retrieved July 1, 2019.
- McGrath M (May 6, 2019). "Nature crisis: Humans 'threaten 1m species with extinction'". BBC. Retrieved July 1, 2019.
- Bar-On, Yinon M; Phillips, Rob; Milo, Ron (2018). "The biomass distribution on Earth". Proceedings of the National Academy of Sciences. 115 (25): 6506–6511. doi:10.1073/pnas.1711842115. PMC 6016768. PMID 29784790.
- Main D (22 November 2013). "7 Iconic Animals Humans Are Driving to Extinction". Live Science.
- Platt JR (25 October 2011). "Poachers Drive Javan Rhino to Extinction in Vietnam". Scientific American. Archived from the original on 6 April 2015.
- Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Di Fiore A, et al. (January 2017). "Impending extinction crisis of the world's primates: Why primates matter". Science Advances. 3 (1): e1600946. Bibcode:2017SciA....3E0946E. doi:10.1126/sciadv.1600946. PMC 5242557. PMID 28116351.
- Fletcher M (January 31, 2015). "Pangolins: why this cute prehistoric mammal is facing extinction". The Telegraph.
- Carrington D (December 8, 2016). "Giraffes facing extinction after devastating decline, experts warn". The Guardian.
- Greenfield, Patrick (September 9, 2020). "Humans exploiting and destroying nature on unprecedented scale – report". The Guardian. Retrieved October 13, 2020.
- McCarthy, Donnachadh (October 1, 2020). "Terrifying wildlife losses show the extinction end game has begun – but it's not too late for change". The Independent. Retrieved October 13, 2020.
- Pennisi E (October 18, 2016). "People are hunting primates, bats, and other mammals to extinction". Science. Retrieved 3 February 2017.
- Ripple WJ, Abernethy K, Betts MG, Chapron G, Dirzo R, Galetti M, et al. (October 2016). "Bushmeat hunting and extinction risk to the world's mammals". Royal Society Open Science. 3 (10): 160498. Bibcode:2016RSOS....360498R. doi:10.1098/rsos.160498. hdl:1893/24446. PMC 5098989. PMID 27853564.
- Williams M, Zalasiewicz J, Haff PK, Schwägerl C, Barnosky AD, Ellis EC (2015). "The Anthropocene Biosphere". The Anthropocene Review. 2 (3): 196–219. doi:10.1177/2053019615591020. S2CID 7771527.
- Morell V (August 11, 2015). "Meat-eaters may speed worldwide species extinction, study warns". Science. Retrieved 3 February 2017.
- Machovina B, Feeley KJ, Ripple WJ (December 2015). "Biodiversity conservation: The key is reducing meat consumption". The Science of the Total Environment. 536: 419–431. Bibcode:2015ScTEn.536..419M. doi:10.1016/j.scitotenv.2015.07.022. PMID 26231772.
- Redford KH (1992). "The empty forest" (PDF). BioScience. 42 (6): 412–422. doi:10.2307/1311860. JSTOR 1311860.
- Peres CA, Nascimento HS (2006). "Impact of Game Hunting by the Kayapo´ of South-eastern Amazonia: Implications for Wildlife Conservation in Tropical Forest Indigenous Reserves". Human Exploitation and Biodiversity Conservation. Topics in Biodiversity and Conservation. 3. pp. 287–313. ISBN 978-1-4020-5283-5. OCLC 207259298.
- Altrichter M, Boaglio G (2004). "Distribution and Relative Abundance of Peccaries in the Argentine Chaco: Associations with Human Factors". Biological Conservation. 116 (2): 217–225. doi:10.1016/S0006-3207(03)00192-7.
- Alverson DL, Freeburg MH, Murawski SA, Pope JG (1996) [1994]. "Bycatch of Marine Mammals". A global assessment of fisheries bycatch and discards. Rome: Food and Agriculture Organization of the United Nations. ISBN 978-92-5-103555-9. OCLC 31424005.
- Glowka L, Burhenne-Guilmin F, Synge H, McNeely JA, Gündling L (1994). IUCN environmental policy and law paper. Guide to the Convention on Biodiversity. International Union for Conservation of Nature. ISBN 978-2-8317-0222-3. OCLC 32201845.
- "About IUCN". International Union for Conservation of Nature. 2014-12-03. Retrieved 3 February 2017.
- Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM (June 2015). "Accelerated modern human-induced species losses: Entering the sixth mass extinction". Science Advances. 1 (5): e1400253. Bibcode:2015SciA....1E0253C. doi:10.1126/sciadv.1400253. PMC 4640606. PMID 26601195.
- Fisher DO, Blomberg SP (April 2011). "Correlates of rediscovery and the detectability of extinction in mammals". Proceedings. Biological Sciences. 278 (1708): 1090–7. doi:10.1098/rspb.2010.1579. PMC 3049027. PMID 20880890.
- Ceballos G, Ehrlich AH, Ehrlich PR (2015). The Annihilation of Nature: Human Extinction of Birds and Mammals. Baltimore: Johns Hopkins University Press. p. 69. ISBN 978-1-4214-1718-9.
- Zhigang J, Harris RB (2008). "Elaphurus davidianus". IUCN Red List of Threatened Species. 2008. Retrieved 2012-05-20.
- McKinney ML, Schoch R, Yonavjak L (2013). "Conserving Biological Resources". Environmental Science: Systems and Solutions (5 ed.). Jones & Bartlett Learning. ISBN 978-1-4496-6139-7. OCLC 777948078.
- Perrin WF, Würsig BG, Thewissen JG (2009). Encyclopedia of marine mammals. Academic Press. p. 404. ISBN 978-0-12-373553-9. OCLC 455328678.
Further reading
- Brown WM (2001). "Natural selection of mammalian brain components". Trends in Ecology and Evolution. 16 (9): 471–473. doi:10.1016/S0169-5347(01)02246-7.
- Jaffa K, Taher NA (2006). "Mammalia Palaestina: The Mammals of Palestine". The Palestinian Biological Bulletin (55): 1–46.
- McKenna MC, Bell SK (1997). Classification of Mammals Above the Species Level. New York: Columbia University Press. ISBN 978-0-231-11013-6. OCLC 37345734.
- Nowak RM (1999). Walker's mammals of the world (6 ed.). Baltimore: Johns Hopkins University Press. ISBN 978-0-8018-5789-8. OCLC 937619124.
- Simpson GG (1945). "The principles of classification and a classification of mammals". Bulletin of the American Museum of Natural History. 85: 1–350.
- Murphy WJ, Eizirik E, O'Brien SJ, Madsen O, Scally M, Douady CJ, et al. (December 2001). "Resolution of the early placental mammal radiation using Bayesian phylogenetics". Science. 294 (5550): 2348–51. Bibcode:2001Sci...294.2348M. doi:10.1126/science.1067179. PMID 11743200. S2CID 34367609.
- Springer MS, Stanhope MJ, Madsen O, de Jong WW (August 2004). "Molecules consolidate the placental mammal tree" (PDF). Trends in Ecology & Evolution. 19 (8): 430–8. doi:10.1016/j.tree.2004.05.006. PMID 16701301.
- Vaughan TA, Ryan JM, Capzaplewski NJ (2000). Mammalogy (4 ed.). Fort Worth, Texas: Saunders College Publishing. ISBN 978-0-03-025034-7. OCLC 42285340.
- Kriegs JO, Churakov G, Kiefmann M, Jordan U, Brosius J, Schmitz J (April 2006). "Retroposed elements as archives for the evolutionary history of placental mammals". PLOS Biology. 4 (4): e91. doi:10.1371/journal.pbio.0040091. PMC 1395351. PMID 16515367.
- MacDonald DW, Norris S (2006). The Encyclopedia of Mammals (3 ed.). London: Brown Reference Group. ISBN 978-0-681-45659-4. OCLC 74900519.
External links
| The Wikibook Dichotomous Key has a page on the topic of: Mammalia |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Mammalia. |
- Biodiversitymapping.org – All mammal orders in the world with distribution maps
- Paleocene Mammals, a site covering the rise of the mammals, paleocene-mammals.de
- Evolution of Mammals, a brief introduction to early mammals, enchantedlearning.com
- European Mammal Atlas EMMA from Societas Europaea Mammalogica, European-mammals.org
- Marine Mammals of the World – An overview of all marine mammals, including descriptions, both fully aquatic and semi-aquatic, noaa.gov
- Mammalogy.org The American Society of Mammalogists was established in 1919 for the purpose of promoting the study of mammals, and this website includes a mammal image library

.jpg.webp)
















