RECOVERY Trial
The Randomised Evaluation of COVID-19 Therapy (RECOVERY Trial or RECOVERY)[1] is a large-enrollment clinical trial of possible treatments for people in the United Kingdom admitted to hospital with severe COVID-19 infection.[2][3][4] As of 2 December 2020, the trial has tested nine interventions on adults: seven repurposed drugs, one newly developed drug and convalescent plasma.[2]

| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|
Treatments
The following treatments are allocated at random to hospitalized people with severe COVID-19 infection:[2][5]
- REGN-COV2 (a cocktail of two anti-viral monoclonal antibodies)
- Aspirin (adults only)
- Colchicine (an anti-inflammatory medication for gout; adults only)
- Baricitinib (an immunomodulatory drug used in rheumatoid arthritis)
Children with PIMS-TS may also be allocated the following:[5]
- Low-dose dexamethasone (a steroid, which reduces inflammation)
- Intravenous immunoglobulin
- Tocilizumab (an anti-inflammatory)
- Anakinra (a drug used to treat rheumatoid arthritis)
The following treatments have previously been included in the trial:
- Lopinavir-Ritonavir (an HIV medication)
- Hydroxychloroquine (used to treat malaria and rheumatism)
- Azithromycin (an antibiotic)
- Convalescent plasma (blood plasma from people who have recovered from COVID-19 and which may contain antibodies against the SARS-CoV-2 virus)
The latter four arms were closed to new entrants after being shown to be ineffective.[6][7][8][9]
Dexamethasone was closed to new adult entries after positive results.
Design
The RECOVERY Trial is a large-scale, randomized controlled trial.[2][10] The primary objective of the trial is to "provide reliable estimates of the effect of study treatments on all-cause mortality at 28 days after first randomisation".[2][10]
It is an "open label" study: people receiving the treatment and the attending clinicians both know which treatment is being administered.[2] It is a multi-arm adaptive clinical trial. New treatments can be added into the trial as it progresses, and other treatment "arms" closed to new enrolment when results have been produced.[4]
The trial protocol was developed in March 2020. The design minimizes the administrative load on hospital staff, who at the time were facing the prospect of overwhelming numbers of COVID-19 admissions.[11][12]
When people who have been hospitalised with COVID-19 are enrolled in the trial, they are automatically randomized to receive trial treatments. If any treatment is contra-indicated (or positively indicated) for that patient, or is not available, then that treatment is not included in the randomization process. The main randomization stage has three parts, so that patients might be allocated none or one or two or three of the trial treatments. If their disease progresses, there may also be a second randomization.[12]
Operations
The trial is run by the Nuffield Departments of Population Health and of Medicine at the University of Oxford.[7][11] The study is led by Peter Horby and Martin Landray who serve as Co-Chief Investigators of the trial.[13][14] By July 2020, the trial was in progress at 176 NHS hospitals in the UK, involving many thousands of health professionals.[15]
The trial began in March 2020 and has an estimated duration through June 2021.[10] As of December 2020, the trial had enrolled more than 20,000 COVID-19 participants admitted to hospitals in the UK.[8]
Results
Dexamethasone
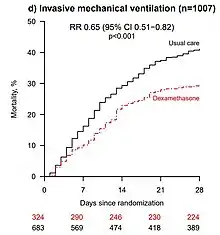
On 22 June 2020, preliminary results were published in a preprint showing that low-dose dexamethasone treatment reduced the death rate by one third in hospitalized people needing ventilators due to severe COVID-19 infection, and by one fifth in people treated with oxygen therapy. There was no benefit (and the possibility of harm) among people who did not require oxygen.[16] The preliminary report was subsequently published in The New England Journal of Medicine.[17]
Impacts
Six days before the pre-print, these results had been announced in a news release.[18] A UK Therapeutic Alert was issued the same day, and all the Chief Medical Officers in the United Kingdom exceptionally recommended an immediate change of UK-wide clinical practice, in advance of publication of any final paper.[19]
Demand for dexamethasone surged after publication of the preprint.[20]
Based on the preliminary, unpublished results of the RECOVERY trial, the US National Institutes of Health COVID-19 Treatment Guidelines Panel recommended dexamethasone in patients with COVID-19 who are on mechanical ventilation or those who require supplemental oxygen, and recommended against dexamethasone for those not requiring supplemental oxygen.[21]
Other countries granted specific approval for the drug as part of standard medical care, among them Japan,[22] Taiwan[23] and South Africa.[24]
A pre-print study, which was awarded Health Data Research UK Open Access Publication of the Month for August 2020, found that the discovery would save approximately 650,000 lives globally over the course of six months.[25][26]
Limitations of dexamethasone result
- John Fletcher, research editor at the BMJ, noted that there were "limitations and causes of concern" in the dexamethasone results.[20]
- Around a third of patients in the trial were still in hospital at the end of the 28-day trial period, so their final outcomes were not known.[20]
- As an immunosuppressive drug, there are fears that dexamethasone could make the illness worse, and prolong the infection in patients where the immune system has not yet overreacted and caused inflammation.[11][27]
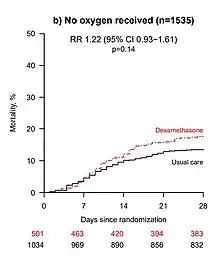
- The preprint authors themselves warned that "the results are consistent with possible harm" in patients who did not require oxygen at the time of enrolment.[16] The trial observed a 22% increase in mortality in these patients (rate ratio 1.22 [95% Confidence Interval 0.93 to 1.61]; p=0.14), though this observation may still be due to chance.[16]
- Responding to the publication, the WHO emphasized that dexamethasone should only be used for patients with severe or critical disease, under close clinical supervision, stating that "There is no evidence this drug works for patients with mild disease or as a preventative measure, and it could cause harm."[28]
Confirmation of dexamethasone result
The results of this initial finding have now been replicated in a systematic review by the World Health Organization's Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group.[29]
Hydroxychloroquine
On 5 June 2020, the trial determined that there was no clinical benefit from use of hydroxychloroquine in people hospitalized with COVID-19.[7] Patients treated with hydroxychloroquine saw a 25.7% 28-day mortality compared to a 23.5% 28-day mortality in those treated with standard care.[30] The trial also showed that hydroxychloroquine was correlated with longer hospital stays and progression to invasive ventilation, but not with cardiac arrhythmia.[30]
Patients received substantial doses of hydroxychloroquine (400 mg every 12h, after two loading doses of 800 mg), higher than other trials.[31] Preprint published on July 15, 2020 stated: "the 4-aminoquinoline drugs are relatively weak antivirals ... Thus, to provide the greatest chance of providing benefit in life threatening COVID-19, the dose regimen was designed to result in rapid attainment and maintenance of plasma concentrations that were as high as safely possible."[30]
Limitations of hydroxychloroquine result
The trial did not address its use as prophylaxis or in patients with less severe SARS-CoV-2 infection managed in the community. The RECOVERY trial was specifically performed in the hospital context and another study by Oxford, the COPCOV trial, has the goal of evaluating hydroxychloroquine as a prophylaxis.[32] Nor did it trial the use of HCQ in combination with azithromycin as has been suggested by some observational studies.[30]
Lopinavir-ritonavir
On 29 June 2020, chief investigators of the trial reported there was no clinical benefit from use of lopinavir-ritonavir in 1,596 people hospitalized with severe COVID-19 infection over 28 days of treatment.[6] These results were published in The Lancet on 5 October 2020.[33]
Azithromycin
In a news release on 14 December 2020, the chief investigators announced that they had found no benefit from azithromycin in patients hospitalised with COVID-19. 2582 patients had received azithromycin, and 5182 were randomised to usual care alone. 28-day mortality in both cohorts was 19%.[8] These preliminary results were released as a preprint on the same day.[34]
Convalescent plasma
In a news release on 15 January 2021, the chief investigators announced the closure of the convalescent plasma arm of the trial. Among 1873 reported deaths from 10,405 patients, the trial reported "no significant difference in the primary endpoint of 28-day mortality (18% convalescent plasma vs. 18% usual care alone; risk ratio 1.04 [95% confidence interval 0.95-1.14]; p=0.34)". The results will be published as soon as possible.[9]
References
- "ISRCTN - ISRCTN50189673: A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus)". www.isrctn.com.
- "RECOVERY Trial". Retrieved 17 June 2020.
- "Coronavirus: Dexamethasone being used to treat NHS patients today". Retrieved 17 June 2020.
- "Biggest COVID-19 trial tests repurposed drugs first". Nature Biotechnology. 38 (5): 510. 11 May 2020. doi:10.1038/s41587-020-0528-x. PMID 32393915. S2CID 218593584. Retrieved 26 June 2020.
- "Recovery Trial Protocol v13" (PDF). Recovery Trial. Retrieved 4 Feb 2021.
- "No clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients studied in RECOVERY" (PDF). RECOVERY Trial: Statement from the Chief Investigators. 29 June 2020. Retrieved 30 June 2020.
- "No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19". Recovery Trial, Nuffield Department of Population Health, University of Oxford, UK. 2020-06-05. Retrieved 2020-06-07.
- Peter Horby; Martin Landray. "RECOVERY trial finds no benefit from azithromycin in patients hospitalised with COVID-19". RECOVERY Trial. Retrieved 14 December 2020.
- RECOVERY trial chief investigators (15 January 2021). "Recovery trial closes recruitment for convalescent plasma treatment for patients hospitalised with COVID-19". Retrieved 15 January 2021.
- Clinical trial number NCT04381936 for "Randomised Evaluation of COVID-19 Therapy (RECOVERY)" at ClinicalTrials.gov
- Fergus Walsh (20 June 2020). "At last some good news about coronavirus". BBC News. Retrieved 20 June 2020.
- "Randomised evaluation of COVID-19 therapy (RECOVERY) - Trial protocol v11" (PDF). RECOVERY Trial. 21 Nov 2020. Retrieved 14 Dec 2020.
- "Preliminary trial results find dexamethasone reduces death in hospitalised patients with severe respiratory complications of COVID-19 - UK Research and Innovation". www.ukri.org. UK Research and Innovation. Retrieved 19 October 2020.
- editor, Sarah Boseley Health (17 June 2020). "Dexamethasone: low-cost drug helps prevent deaths of sickest coronavirus patients". The Guardian.CS1 maint: extra text: authors list (link)
- KupferschmidtJul. 2, Kai (2 July 2020). "One U.K. trial is transforming COVID-19 treatment. Why haven't others delivered more results?". Science. Retrieved 19 October 2020.
- RECOVERY Collaborative Group (2020). "Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report". MedRXiv. doi:10.1101/2020.06.22.20137273. S2CID 219965377. Retrieved 23 June 2020.
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. (July 2020). "Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report". New England Journal of Medicine. doi:10.1056/NEJMoa2021436. PMC 7383595. PMID 32678530.
- "Dexamethasone reduces death in hospitalised patients with severe respiratory complications of COVID-19". University of Oxford. 16 June 2020. Retrieved 16 June 2020.
- "Dexamethasone in COVID-19" (PDF). 16 June 2020. Retrieved 23 June 2020.
- Mahase E (June 2020). "Covid-19: Demand for dexamethasone surges as RECOVERY trial publishes preprint". BMJ. 369: m2512. doi:10.1136/bmj.m2512. PMID 32576548.
- "The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19". NIH COVID-19 Treatment Guidelines. National Institutes of Health. 25 June 2020. Retrieved 26 June 2020.
- hermesauto (2020-07-22). "Japan approves dexamethasone as coronavirus treatment". The Straits Times. Retrieved 2020-09-06.
- "Taiwan provisionally approves dexamethasone as coronavirus treatment". Reuters. 2020-08-04. Retrieved 2020-09-06.
- "South Africa approves dexamethasone to treat COVID-19".
- "The potential health and economic impact of dexamethasone treatment for patients with COVID-19". HDR UK. Retrieved 2020-09-06.
- Aguas, Ricardo; Mahdi, Adam; Shretta, Rima; Horby, Peter; Landray, Martin; White, Lisa J. (2020-07-30). "The potential health and economic impact of dexamethasone treatment for patients with COVID-19". MedRxiv: 2020.07.29.20164269. doi:10.1101/2020.07.29.20164269. S2CID 220846974.
- Roni Caryn Rabin (24 June 2020). "Breakthrough Drug for Covid-19 May Be Risky for Mild Cases". New York Times. Retrieved 25 June 2020.
- "WHO Director-General's opening remarks at the media briefing on COVID-19 - 22 June 2020". World Health Organization. Retrieved 25 June 2020.
- Group, The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working; Sterne, Jonathan A. C.; Murthy, Srinivas; Diaz, Janet V.; Slutsky, Arthur S.; Villar, Jesús; Angus, Derek C.; Annane, Djillali; Azevedo, Luciano Cesar Pontes; Berwanger, Otavio; Cavalcanti, Alexandre B. (2020-09-02). "Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis". JAMA. 324 (13): 1330–1341. doi:10.1001/jama.2020.17023. PMC 7489434. PMID 32876694.
- Horby, Peter, et al. “Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary Results from a Multi-Centre, Randomized, Controlled Trial.” MedRxiv, Cold Spring Harbor Laboratory Press, 1 Jan. 2020,
- http://www.francesoir.fr/oxford-recovery-hospital-test-overdose-hard-pills-swallow
- "Chloroquine/ Hydroxychloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting; a Randomised, Placebo-controlled Prophylaxis Study (COPCOV)". clinicaltrials.gov. 1 October 2020. Retrieved 19 October 2020.
- Horby, Peter W.; Mafham, Marion; Bell, Jennifer L.; Linsell, Louise; Staplin, Natalie; Emberson, Jonathan; Palfreeman, Adrian; Raw, Jason; Elmahi, Einas; Prudon, Benjamin; Green, Christopher; Carley, Simon; Chadwick, David; Davies, Matthew; Wise, Matthew P.; Baillie, J Kenneth; Chappell, Lucy C.; Faust, Saul N.; Jaki, Thomas; Jefferey, Katie; Lim, Wei Shen; Montgomery, Alan; Rowan, Kathryn; Juszczak, Edmund; Haynes, Richard; Landray, Martin J. (5 October 2020). "Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial". The Lancet. 396 (10259): 1345–1352. doi:10.1016/S0140-6736(20)32013-4. PMC 7535623. PMID 33031764. Retrieved 1 December 2020.
- "Azithromycin in Hospitalised Patients with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial". 14 December 2020. doi:10.1101/2020.12.10.20245944. Retrieved 14 December 2020.