Pertechnetic acid
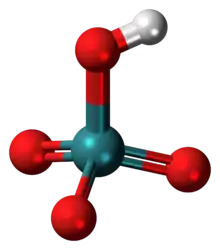
Pertechnetic acid (HTcO4) is a compound of technetium that is produced by reacting technetium(VII) oxide (Tc2O7) with water or strong oxidizing acids, such as nitric acid, concentrated sulfuric acid, aqua regia.[1] The dark red hygroscopic substance is a strong acid, with a pKa of 0.32,[2] as such it exists almost entirely as the pertechnetate ion in aqueous solution.
 | |
| Names | |
|---|---|
| IUPAC name
Pertechnetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| HO4Tc | |
| Molar mass | 163 g·mol−1 |
| Conjugate base | Pertechnetate |
| Related compounds | |
Other anions |
Permanganic acid Perrhenic acid |
Other cations |
Sodium pertechnetate |
Related compounds |
Perchloric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use of strong enough acid solution, for example, concentrated sulfuric acid, can generate the protonated form, which then exists as the octahedral TcO3(OH)(H2O)2 dihydrate complex.[3]
See also
References
- Schwochau, Klaus (2000). Technetium : Chemistry and radiopharmaceutical applications. Weinheim [u.a.]: Wiley-VCH. p. 127. ISBN 3-527-29496-1.
- Omori, T.; Asahina, K.; Suganuma, H. (1995). "Mechanism of the solvent extraction of pertechnetate with tetraphenylarsonium chloride". Journal of Radioanalytical and Nuclear Chemistry Articles. 191 (1): 99–104. doi:10.1007/BF02035989.
- Poineau F, Weck PF, German K, Maruk A, Kirakosyan G, Lukens W, Rego DB, Sattelberger AP, Czerwinski KR (2010). "Speciation of heptavalent technetium in sulfuric acid: structural and spectroscopic studies" (PDF). Dalton Transactions. 39 (37): 8616–8619. doi:10.1039/C0DT00695E. PMID 20730190.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.