Halazone
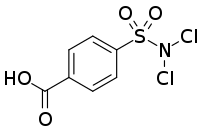
Halazone (4-((dichloroamino)sulfonyl)benzoic acid) is a chemical compound whose formula can be written as either C
7H
5Cl
2NO
4S or (HOOC)(C
6H
4)(SO
2)(NCl
2). It has been widely used to disinfect drinking water.
 | |
| Names | |
|---|---|
| IUPAC name
4-((Dichloroamino)sulfonyl)benzoic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.140 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1479 |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C7H5Cl2NO4S | |
| Molar mass | 270.08 g·mol−1 |
| Appearance | Fine white powder with an odor of chlorine[2] |
| Melting point | 213 °C (415 °F; 486 K);[3] 196 °C with decomposition.[4] |
| Less than 1 g/L at 70 °F [2] | |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Other names for this compound include p-sulfondichloramidobenzoic acid, 4-(dichlorosulfamoyl)benzoic acid, and Pantocide.
Uses
Halazone tablets have been used to disinfect water for drinking, especially where treated tap water is not available. A typical dosage is 4 mg/L.[5][6]
Halazone tablets were commonly used during World War II by U.S. soldiers for portable water purification, even being included in accessory packs for C-rations until 1945.[7]
Halazone has largely been replaced in that use by sodium dichloroisocyanurate. The primary limitation of halazone tablets was the very short usable life of opened bottles, typically 3 days or less, unlike iodine-based tablets which have a usable open bottle life of three months.
Dilute halazone solutions (4 to 8 ppm of available chlorine) has also been used to disinfect contact lenses,[8] and as a spermicide.
Mechanism of action
Halazone's disinfecting activity is mainly due to the hypochlorous acid (HClO) released by hydrolysis of the chlorine-nitrogen bonds when the product is dissolved in water:[8]
- (R1)(R2)NCl + H
2O → HOCl + (R1)(R2)NH
The hypochlorous acid is a powerful oxidizer and chlorinating agent that destroys or denatures many organic compounds.
Production
Halazone can be prepared by chlorination of p-sulfonamidobenzoic acid.[4]
Another synthesis route is the oxidation of dichloramine-T with potassium permanganate in a mild alkaline medium.[4]
See also
- Bleach
- Chlorine-releasing compound
- Chloramine-T (tosylchloramide sodium salt), another water disinfection agent.
- Water chlorination
References
- PubChem: "Halazone". Accessed on 2018-06-18.
- NTP (1992), cited by PubChem
- Jean-Claude Bradley: Open Melting Point Dataset. Quoted by Chemspider.
- Saljoughian, M.; Sadeghi, M. T. (1986). "An improved procedure for the synthesis ofp-(dichlorosulfamoyl)benzoic acid (Halazone)". Monatshefte für Chemie. 117 (4): 553. doi:10.1007/BF00810903.
- Gripo Laboratories: "Water purification range: Halazone USP based Chlorine Tablets". Product page, accessed on 2018-06-18
- Precise Health Care PVT LTD: "Halazone tablets". Product page, accessed on 2018-06-18
- Hlavatá, L; Aguilaniu, H; Pichová, A; Nyström, T (2003). "The oncogenic RAS2val19 mutation locks respiration, independently of PKA, in a mode prone to generate ROS". The EMBO Journal. 22 (13): 3337–3345. doi:10.1093/emboj/cdg314. PMC 165639. PMID 12839995.
- Rosenthal, Ruth Ann; Schlitzen, Ronald L; McNamee, Linda S; Dassanayake, Nissanake L; Amass, Roger (1992). "Antimicrobial activity of organic chlorine releasing compounds". Journal of the British Contact Lens Association. 15 (2): 81. doi:10.1016/0141-7037(92)80044-Z.