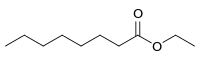
Ethyl octanoate
Ethyl octanoate, also known as ethyl caprylate, is a fatty acid ester formed from caprylic acid and ethanol. It has the semi-developed formula of CH3(CH2)6COOCH2CH3, and is used in food industries as a flavoring and in the perfume industry as a scent additive. It is present in many fruits and alcoholic beverages, and has a strong odor of fruit and flowers. It is used in the creation of synthetic fruity scents.[5]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl octanoate | |
| Other names
Ethyl caprylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.078 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H20O2 | |
| Molar mass | 172.268 g·mol−1 |
| Density | 0.86215 g/cm3[1] |
| Melting point | −48 °C (−54 °F; 225 K)[2] |
| Boiling point | 208 °C (406 °F; 481 K)[2] |
| 70.1 mg/L [3][4] | |
| Vapor pressure | 0.2 mbar at 20°C; 3.18 mbar at 60°C [2] |
| Viscosity | 1.411 mPa·s[1] |
| Hazards | |
| Flash point | 79°C [2] |
| 325°C [2] | |
| Explosive limits | 0.7 - Vol.% [2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
25.96 g/kg (rat, oral) [3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Sheu, Yaw-Wen; Tu, Chein-Hsiun (2006). "Densities and Viscosities of Binary Mixtures of Isoamyl Acetate, Ethyl Caproate, Ethyl Benzoate, Isoamyl Butyrate, Ethyl Phenylacetate, and Ethyl Caprylate with Ethanol at T = (288.15, 298.15, 308.15, and 318.15) K". Journal of Chemical and Engineering Data. 51 (2): 496–503. doi:10.1021/je050389b.
- Record of Ethyl octanoate in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 10 August 2010.
- Ethyl caprylate in the ChemIDplus database, accessed 10 August 2010
- Mark, James E. (2007). Physical Properties of Polymer Handbook (2nd ed.). Springer. p. 294. ISBN 978-0387690025. Retrieved 1 March 2013.
- Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (15 January 2003). "Flavors and Fragrances". Flavors and Flagrances. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. doi:10.1002/14356007.a11_141. ISBN 3527306730.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.