Chidamide
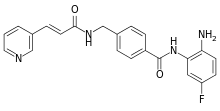
Chidamide (Epidaza) is a histone deacetylase inhibitor (HDI) developed in China.[1] It was also known as HBI-8000.[2] It is a benzamide HDI and inhibits Class I HDAC1, HDAC2, HDAC3, as well as Class IIb HDAC10.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Epidaza |
| Other names | Tucidinostat |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C22H19FN4O2 |
| Molar mass | 390.418 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Chidamide is approved by the Chinese FDA for relapsed or refractory peripheral T-cell lymphoma (PTCL), and has orphan drug status in Japan.[2] As of April 2015 it is only approved in China.[1]
Chidamide is being researched as a treatment for pancreatic cancer.[4][5][6] However, it is not US FDA approved for the treatment of pancreatic cancer.
References
- Lowe D (April 2015). "China's First Homegrown Pharma". Seeking Alpha.
- "Chipscreen Biosciences Announces CFDA Approval of Chidamide (Epidaza) for PTCLs in China". PR Newswire Association LLC.
- "HUYA Bioscience International Grants An Exclusive License For HBI-8000 In Japan And Other Asian Countries To Eisai". PR Newswire Association LLC. February 2016.
- Qiao Z, Ren S, Li W, Wang X, He M, Guo Y, et al. (April 2013). "Chidamide, a novel histone deacetylase inhibitor, synergistically enhances gemcitabine cytotoxicity in pancreatic cancer cells". Biochemical and Biophysical Research Communications. 434 (1): 95–101. doi:10.1016/j.bbrc.2013.03.059. PMID 23541946.
- Guha M (April 2015). "HDAC inhibitors still need a home run, despite recent approval". Nature Reviews. Drug Discovery. 14 (4): 225–6. doi:10.1038/nrd4583. PMID 25829268. S2CID 36758974.
- Wang SS (2015-04-02). "A New Cancer Drug, Made in China". The Wall Street Journal. Retrieved 13 April 2015.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.