Acrosin
Acrosin is a digestive enzyme that acts as a protease. In humans, acrosin is encoded by the ACR gene.[1][2] Acrosin is released from the acrosome of spermatozoa as a consequence of the acrosome reaction. It aids in the penetration of the Zona Pellucida.
Enzyme Mechanism
Acrosin is a typical serine proteinase with trypsin-like specificity.[3]
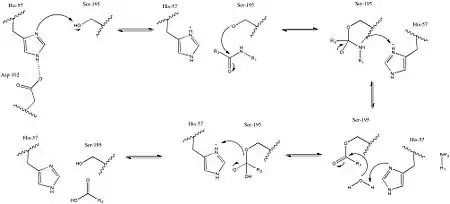
The reaction proceeds according to the usual serine protease mechanism. First, His-57 deprotonates Ser-195, allowing it to serve as a nucleophile. Deprotonated Ser-195 then reacts with the carbonyl carbon of a peptide, forming a tetrahedral intermediate. The tetrahedral intermediate then collapses, resulting in an H2N-R1 leaving group, which is protonated through His-57. Finally, His-57 deprotonates a water molecule, which can then serve as a nucleophile by similarly reacting with the carbonyl carbon. Collapse of the tetrahedral intermediate then results in a Ser-195 leaving group, which is protonated through His-57, resulting in all residues returned to their pre-catalytic state, and a carboxylic acid where there was previously a peptide bond.
Biological Function
Acrosin is the major proteinase present in the acrosome of mature spermatozoa. It is stored in the acrosome in its precursor form, proacrosin. Upon stimulus, the acrosome releases its contents onto the zona pellucida. After this reaction occurs, the zymogen form of the protease is then processed into its active form, β-acrosin. The active enzyme then functions in the lysis of the zona pellucida, thus facilitating penetration of the sperm through the innermost glycoprotein layers of the ovum.[3]
The importance of acrosin in the acrosome reaction has been contested. It has been found through genetic knockout experiments that mouse spermatozoa lacking β-acrosin (the active protease) still have the ability to penetrate the zona pellucida.[4] Thus, some argue for its role in assisting in the dispersal of acrosomal contents following the acrosome reaction, while others demonstrate evidence for its role as a secondary binding protein between the spermatozoa and zona pellucida.[5][6][7] Under the secondary binding protein hypothesis, acrosin could serve a role in binding to molecules on the zona pellucida, tethering the spermatozoa to the egg. This "tethering" would ensure penetration due to the applied motile force of the spermatozoa.[8]
Acrosin regulation has been found to occur through protein C inhibitor (PCI). PCI is present in the male reproductive tract at 40x higher concentrations than in blood plasma.[9] PCI has been demonstrated to inhibit the proteolytic activity of acrosin.[9] Thus, PCI has been hypothesized to have a protective role: if acrosomal enzymes were released prematurely, or if the spermatozoa was degenerated within the male reproductive tract, the high concentrations of PCI would inhibit acrosin from inflicting proteolytic damage on nearby tissues.[10]
Structure
β-acrosin demonstrates a high degree of sequence identity (70-80%) between boar, bull, rat, guinea pig, mouse, and human isoforms.[3] There exists a somewhat similar (27-35%) sequence identity between β-acrosin and other serine proteases such as trypsin and chymotrypsin.[3] While most serine proteases are activated through one cleavage event, proacrosin requires processing at both the N and C-terminal domains. Proacrosin is first cleaved between Arg-22 and adjacent Valine to create a 22 residue light chain, and an active protease termed α-acrosin.[3] This light chain remains associated with the heavy chain, cross-linked through two disulfide bonds to form a heterodimer. Following these N-terminal cleavage events, three cleavages at the C-terminal domain removes 70 residues, yielding β-acrosin.[3] Acrosin has two sites which have been identified as possible N-glycosylation sites: Asn-2 and Asn-169.[3]

The catalytic triad consists of residues His-57, Asp-102, and Ser-195.[3] These residues are found in a binding pocket that has been termed the "S1" pocket, consistent with the naming scheme that has been adopted for other proteases.[11] The S1 pocket regulates acrosin's specificity for Arg and Lys substrates, with a conserved Trp-215 serving as a "gatekeeper" residue for the binding site entrance.[3]
An important structural element of β-acrosin is a highly charged patch (formed through both amino acids and post-translational modifications) on its surface region, that has been termed the "anion binding exosite."[3] This site consists of an area of excess positive charge, which has been hypothesized to be important in binding to the matrix of the zona pellucida, a heavily glycosylated and sulfated region with excess negative charge.[12] This structural feature is consistent with the secondary binding protein hypothesis, as charge-charge interactions would stabilize a protein-zona pellucida "tethering" complex.[13] Further consistent with this structural hypothesis is the knowledge that suramin - a polysulfated drug (with substantial corresponding negative charge) has been found to inhibit sperm-zona pellucida binding.[14]
Disease and Pharmaceutical Relevance
While one study which utilized mice models indicated that acrosin is not a necessary component of zona pellucida penetration, other studies in humans have shown an association between low acrosomal proteinase activity and infertility.[15][16] Other research groups have demonstrated a significant correlation between acrosin activity and sperm motility.[17] In rabbit models, an intravaginal contraceptive device that secreted tetradecyl sodium sulfate, a known inhibitor of acrosin and hyaluronidases, had a complete contraceptive effect.[18] Although its exact mechanism of action is not entirely clear, acrosin could thus serve as a novel target for contraceptive agents. Acrosin may represent as a uniquely druggable target due to its location and high cellular specificity.[19] Thus, developing inhibitors of acrosin could provide the basis for safe, reversible male contraceptives, or female contraceptives through the use of intravaginal contraceptive devices.[19]
Moreover, as serine proteases are important in the potentiation of HIV, research has found that an acrosin inhibitor, 4'-acetamidophenyl 4-guanidinobenzoate, possess the ability to inhibit HIV infection in virus-inoculated lymphocytes.[20] This suggests the further role of acrosin inhibitors as potentially viable agents in the prevention of HIV transmission.[20]
References
- Adham IM, Klemm U, Maier WM, Engel W (Jan 1990). "Molecular cloning of human preproacrosin cDNA". Human Genetics. 84 (2): 125–8. doi:10.1007/bf00208925. PMID 2298447.
- Honda A, Siruntawineti J, Baba T (2002). "Role of acrosomal matrix proteases in sperm-zona pellucida interactions". Human Reproduction Update. 8 (5): 405–12. doi:10.1093/humupd/8.5.405. PMID 12398221.
- Tranter, Rebecca; Read, Jon A.; Jones, Roy; Brady, R. Leo (2000-11-15). "Effector Sites in the Three-Dimensional Structure of Mammalian Sperm β-Acrosin". Structure. 8 (11): 1179–1188. doi:10.1016/S0969-2126(00)00523-2. ISSN 0969-2126. PMID 11080640.
- T. Baba, S. Azuma, S. Kashiwabara, Y. Toyoda. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization" J. Biol. Chem. 1994; 269 , pp. 31845–31849
- K. Yamagata, T. Baba, et al. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction" J. Biol. Chem. 1998; 273 , pp. 10470–10474
- R. Jones, C.R. Brown. Identification of a zona-binding protein from boar spermatozoa as proacrosin. Expl" Cell Res 1987; 171 , pp. 505–508
- R. Jones. Interaction of zona pellucida glycoproteins, sulphated carbohydrates and synthetic polymers with proacrosin, the putative egg-binding protein from mammalian spermatozoa" Development 1991; 111 , pp. 1155–1163
- D.P. Green. The head shapes of some mammalian spermatozoa and their possible relationship to the shape of the penetration slit through the zona pellucida. J. Reprod. Fertil., 83 (1988), pp. 377–387
- Laurell, M; Christensson, A; Abrahamsson, PA; Stenflo, J; Lilja, H (1992). "Protein C inhibitor in human body fluids. Seminal plasma is rich in inhibitor antigen deriving from cells throughout the male reproductive system". J Clin Invest. 89 (4): 1094–101. doi:10.1172/JCI115689. PMC 442965. PMID 1372913.
- Zheng, X; Geiger, M; Ecke, S (1994). "Inhibition of acrosin by protein C inhibitor and localization of protein C inhibitor to spermatozoa". Am. J. Physiol. 267 (2 Pt 1): C466–72. doi:10.1152/ajpcell.1994.267.2.C466. PMID 7521127.
- I. Schechter, A. Berger. On the size of the active site in proteases. I. Papain. Biochim. Biophys. Res. Commun, 27 (1967), pp. 157–162.
- Nakano M, Tobets T, et al. (1990). "Further fractionation of the glycoprotein families of porcine zona pellucida by anion exchange HPLC and some characterization of the separated fractions". J. Biochem. 107: 144–150. doi:10.1093/oxfordjournals.jbchem.a122998.
- S. Shimizu, M. Tsuji, J. Dean. In vitro biosynthesis of three sulphated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture" J. Biol. Chem. 1983; 258, pp. 5858–5863
- Jones R, Parry R, Leggio LL, Nickel P (1996). "Inhibition of sperm-zona binding by suramin, a potential "lead" compound for design of new anti-fertility agents". Mol. Hum. Rep. 2 (8): 597–605. doi:10.1093/molehr/2.8.597.
- Welker B, Bernstein GS, Diedrich K, Nakamura RM, Krebs D (Oct 1988). "Acrosomal proteinase activity of human spermatozoa and relation of results to semen quality". Hum Reprod. 3 (Suppl 2): 75–80. doi:10.1093/humrep/3.suppl_2.75.
- Tummon I.S.; Yuzpe A.A.; Daniel S.A.; Deutsch A. Total acrosin activity correlates with fertility potential after fertilization in vitro" Fertil Steril 1991 Nov;56(5):933-8.
- Cui YH, Zhao RL, Wang Q, Zhang ZY (Sep 2000). "Determination of sperm acrosin activity for evaluation of male fertility". Asian J Androl. 2: 229–232.
- Burck P.J., Zimmerman R.E. An intravaginal contraceptive device for the delivery of an acrosin and hyaluronidase inhibitor" Fertil Steril 1984 Feb;41(2):314-8.
- Ning, Weiwei; Zhu, Ju; Zheng, Canhui; Liu, Xuefei; Song, Yunlong; Zhou, Youjun; Zhang, Xiaomeng; Zhang, Ling; Sheng, Chunquan (2013-04-01). "Fragment-Based Design of Novel Quinazolinon Derivatives as Human Acrosin Inhibitors". Chemical Biology & Drug Design. 81 (4): 437–441. doi:10.1111/cbdd.12106. ISSN 1747-0285. PMID 23331539.
- Bourinbaiar AS, Lee-Huang S (May 1995). "Acrosin inhibitor, 4'-acetamidophenyl 4-guanidinobenzoate, an experimental vaginal contraceptive with anti-HIV activity". Contraception. 51 (5): 319–22. doi:10.1016/0010-7824(95)00094-q. PMID 7628208.
Further reading
- Elce JS, McIntyre EJ (Jan 1982). "Purification of bovine and human acrosin". Canadian Journal of Biochemistry. 60 (1): 8–14. doi:10.1139/o82-002. PMID 6802470.
- Kimura K, Wakamatsu A, Suzuki Y, Ota T, Nishikawa T, Yamashita R, Yamamoto J, Sekine M, Tsuritani K, Wakaguri H, Ishii S, Sugiyama T, Saito K, Isono Y, Irie R, Kushida N, Yoneyama T, Otsuka R, Kanda K, Yokoi T, Kondo H, Wagatsuma M, Murakawa K, Ishida S, Ishibashi T, Takahashi-Fujii A, Tanase T, Nagai K, Kikuchi H, Nakai K, Isogai T, Sugano S (Jan 2006). "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes". Genome Research. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.
- Klemm U, Müller-Esterl W, Engel W (Oct 1991). "Acrosin, the peculiar sperm-specific serine protease". Human Genetics. 87 (6): 635–41. doi:10.1007/bf00201716. PMID 1937464.
- Kim J, Bhinge AA, Morgan XC, Iyer VR (Jan 2005). "Mapping DNA-protein interactions in large genomes by sequence tag analysis of genomic enrichment". Nature Methods. 2 (1): 47–53. doi:10.1038/nmeth726. PMID 15782160.
- Moreno RD, Hoshi M, Barros C (May 1999). "Functional interactions between sulphated polysaccharides and proacrosin: implications in sperm binding and digestion of zona pellucida". Zygote. 7 (2): 105–11. doi:10.1017/S0967199499000453. PMID 10418103.
- Liu RZ, Lu YL, Xu ZG, Zuo WJ, Xin JL, Wang ZS (2003). "[The effect of semen antisperm antibody on human sperm acrosin activity]". Zhonghua Nan Ke Xue = National Journal of Andrology. 9 (4): 252–3. PMID 12931362.
- Steven FS, Griffin MM, Chantler EN (Aug 1982). "Inhibition of human and bovine sperm acrosin by divalent metal ions. Possible role of zinc as a regulator of acrosin activity". International Journal of Andrology. 5 (4): 401–12. doi:10.1111/j.1365-2605.1982.tb00270.x. PMID 6815104.
- Marí SI, Rawe V, Biancotti JC, Charreau EH, Dain L, Vazquez-Levin MH (Jun 2003). "Biochemical and molecular studies of the proacrosin/acrosin system in patients with unexplained infertility". Fertility and Sterility. 79 Suppl 3: 1676–9. doi:10.1016/s0015-0282(03)00372-8. PMID 12801583.
- Glogowski J, Demianowicz W, Piros B, Ciereszko A (Oct 1998). "Determination of acrosin activity of boar spermatozoa by the clinical method: optimization of the assay and changes during short-term storage of semen". Theriogenology. 50 (6): 861–72. doi:10.1016/S0093-691X(98)00191-5. PMID 10734459.
- Furlong LI, Veaute C, Vazquez-Levin MH (Jun 2005). "Binding of recombinant human proacrosin/acrosin to zona pellucida glycoproteins. II. Participation of mannose residues in the interaction". Fertility and Sterility. 83 (6): 1791–6. doi:10.1016/j.fertnstert.2004.12.043. PMID 15950652.
- Furlong LI, Harris JD, Vazquez-Levin MH (Jun 2005). "Binding of recombinant human proacrosin/acrosin to zona pellucida (ZP) glycoproteins. I. Studies with recombinant human ZPA, ZPB, and ZPC". Fertility and Sterility. 83 (6): 1780–90. doi:10.1016/j.fertnstert.2004.12.042. PMID 15950651.
- Hartley JL, Temple GF, Brasch MA (Nov 2000). "DNA cloning using in vitro site-specific recombination". Genome Research. 10 (11): 1788–95. doi:10.1101/gr.143000. PMC 310948. PMID 11076863.
- Collins JE, Wright CL, Edwards CA, Davis MP, Grinham JA, Cole CG, Goward ME, Aguado B, Mallya M, Mokrab Y, Huckle EJ, Beare DM, Dunham I (2004). "A genome annotation-driven approach to cloning the human ORFeome". Genome Biology. 5 (10): R84. doi:10.1186/gb-2004-5-10-r84. PMC 545604. PMID 15461802.
- Dubé C, Leclerc P, Baba T, Reyes-Moreno C, Bailey JL (2005). "The proacrosin binding protein, sp32, is tyrosine phosphorylated during capacitation of pig sperm". Journal of Andrology. 26 (4): 519–28. doi:10.2164/jandrol.04163. PMID 15955892.
- Zahn A, Furlong LI, Biancotti JC, Ghiringhelli PD, Marijn-Briggiler CI, Vazquez-Levin MH (Mar 2002). "Evaluation of the proacrosin/acrosin system and its mechanism of activation in human sperm extracts". Journal of Reproductive Immunology. 54 (1–2): 43–63. doi:10.1016/S0165-0378(01)00080-8. PMID 11839395.
- Howes E, Pascall JC, Engel W, Jones R (Nov 2001). "Interactions between mouse ZP2 glycoprotein and proacrosin; a mechanism for secondary binding of sperm to the zona pellucida during fertilization". Journal of Cell Science. 114 (Pt 22): 4127–36. PMID 11739644.
- Yudin AI, Vandevoort CA, Li MW, Overstreet JW (Jul 1999). "PH-20 but not acrosin is involved in sperm penetration of the macaque zona pellucida". Molecular Reproduction and Development. 53 (3): 350–62. doi:10.1002/(SICI)1098-2795(199907)53:3<350::AID-MRD11>3.0.CO;2-9. PMID 10369396.
External links
- The MEROPS online database for peptidases and their inhibitors: S01.223
- Acrosin at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
