Zirconocene dichloride
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air.
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.013.697 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H10Cl2Zr | |||
| Molar mass | 292.31 g·mol−1 | ||
| Appearance | white solid | ||
| Soluble (Hydrolysis) | |||
| Hazards | |||
| Safety data sheet | CAMEO Chemicals MSDS | ||
| Related compounds | |||
Related compounds |
Titanocene dichloride Hafnocene dichloride Vanadocene dichloride Niobocene dichloride Tanatalocene dichloride Tungstenoocene dichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and structure
Zirconocene dichloride may be prepared from zirconium(IV) chloride-THF complex and sodium cyclopentadienide:
- ZrCl4(THF)2 + 2 NaCp → Cp2ZrCl2 + 2 NaCl + 2 THF
The closely related compound Cp2ZrBr2 was first described by Birmingham and Wilkinson.[1]
The compound is a bent metallocene: the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 128°. The Cl-Zr-Cl angle of 97.1° is wider than in niobocene dichloride (85.6°) and molybdocene dichloride (82°). This trend helped to establish the orientation of the HOMO in this class of complex.[2]
Reactions
Schwartz's reagent
Zirconocene dichloride reacts with lithium aluminium hydride to give Cp2ZrHCl Schwartz's reagent:
- (C5H5)2ZrCl2 + 1/4 LiAlH4 → (C5H5)2ZrHCl + 1/4 LiAlCl4
Since lithium aluminium hydride is a strong reductant, some over-reduction occurs to give the dihydrido complex, Cp2ZrH2; treatment of the product mixture with methylene chloride converts it to Schwartz's reagent.[3]
Negishi reagent
Zirconocene dichloride can also be used to prepare the Negishi reagent, Cp2Zr(η2-butene), which can be used as a source of Cp2Zr in oxidative cyclisation reactions. The Negishi reagent is prepared by treating zirconocene dichloride with n-BuLi, leading to replacement of the two chloride ligands with butyl groups. The dibutyl compound subsequently undergoes beta-hydride elimination to give one η2-butene ligand, with the other butyl ligand promptly lost as butane via reductive elimination.[4]

Carboalumination
Zirconocene dichloride catalyzes the carboalumination of alkynes by trimethylaluminum to give a (alkenyl)dimethylalane, a versatile intermediate for further cross coupling reactions for the synthesis of stereodefined trisubstituted olefins. For example, α-farnesene can be prepared as a single stereoisomer by carboalumination of 1-buten-3-yne with trimethylaluminum, followed by palladium-catalyzed coupling of the resultant vinylaluminum reagent with geranyl chloride.[5]
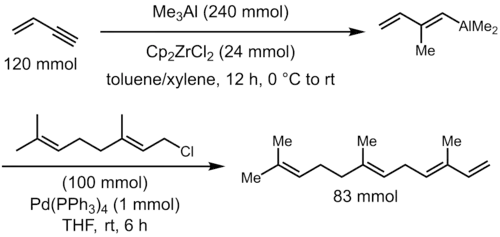
The use of trimethylaluminum for this reaction results in exclusive formation of the syn-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminum reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities.[6] Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
References
- G. Wilkinson and J. M. Birmingham (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- K. Prout, T. S. Cameron, R. A. Forder, and in parts S. R. Critchley, B. Denton and G. V. Rees "The crystal and molecular structures of bent bis-π-cyclopentadienyl-metal complexes: (a) bis-π-cyclopentadienyldibromorhenium(V) tetrafluoroborate, (b) bis-π-cyclopentadienyldichloromolybdenum(IV), (c) bis-π-cyclopentadienylhydroxomethylaminomolybdenum(IV) hexafluorophosphate, (d) bis-π-cyclopentadienylethylchloromolybdenum(IV), (e) bis-π-cyclopentadienyldichloroniobium(IV), (f) bis-π-cyclopentadienyldichloromolybdenum(V) tetrafluoroborate, (g) μ-oxo-bis[bis-π-cyclopentadienylchloroniobium(IV)] tetrafluoroborate, (h) bis-π-cyclopentadienyldichlorozirconium" Acta Crystallogr. 1974, volume B30, pp. 2290–2304. doi:10.1107/S0567740874007011
- S. L. Buchwald; S. J. LaMaire; R. B.; Nielsen; B. T. Watson; S. M. King. "Schwartz's Reagent". Organic Syntheses.; Collective Volume, 9, p. 162
- Negishi, E.; Takashi, T. (1994). "Patterns of Stoichiometric and Catalytic Reactions of Organozirconium and Related Complexes of Synthetic Interest". Accounts of Chemical Research. 27 (5): 124–130. doi:10.1021/ar00041a002.
- "Palladium-Catalyzed Synthesis of 1,4-Dienes by Allylation of Alkenylalanes: α-Farnesene". www.orgsyn.org. Retrieved 2019-11-27.
- Huo, Shouquan (2016-09-19), Rappoport, Zvi (ed.), "Carboalumination Reactions", PATAI'S Chemistry of Functional Groups, Chichester, UK: John Wiley & Sons, Ltd, pp. 1–64, doi:10.1002/9780470682531.pat0834, ISBN 978-0-470-68253-1, retrieved 2021-01-19
Further reading
- A. Maureen Rouhi (1998). "Organozirconium Chemistry Arrives". Chemical & Engineering News. 82 (16): 162. doi:10.1021/cen-v082n015.p035.