Varanus (Odatria)
The subgenus Odatria, sometimes known as the dwarf monitor lizards,[1] consists of small monitor lizards found in Australia and Indonesia. Species in this subgenus include the smallest monitor species in the world, the tiny 16 gram Dampier Peninsula monitor, but also includes some more medium sized species such as the 240 gram black-palmed rock monitor. [2]
| Odatria | |
|---|---|
 | |
| Spiny-tailed monitor (Varanus acanthurus) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Family: | Varanidae |
| Genus: | Varanus |
| Subgenus: | Odatria Gray, 1838 |
| Type species | |
| Varanus tristis Schlegel, 1839 | |
| Species | |
| |
Taxonomy
Odatria was coined by John Edward Gray in 1838, albeit as a genus name.
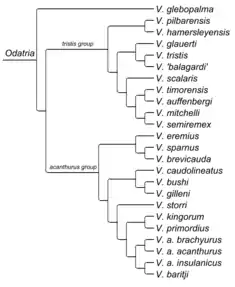
Odatria is the most species diverse subgenus of monitor lizards, with 22 different species. The subgenus also includes two species complexes represented by the spiny-tailed monitor and the Timor monitor. Alternatively, Vidal et al. 2012 splits Odatria into two species groups represented by the spiny-tailed monitor and the black-headed monitor.
In the past, tree monitors such as the green tree monitor have sometimes been included within Odatria as well as Euprepriosaurus, but now form their own subgenus Hapturosaurus.
A 2020 phylogenomic study by Brennan et al. found that Odatria is most closely related to much larger Australian monitor lizards from the subgenus Varanus, which includes the largest living lizard, the Komodo dragon, as well as the monotypic subgenus Papusaurus of which the crocodile monitor is the only member of. Together, Odatria, Varanus, and Papusaurus form a monophyletic clade of Indo-Australopapuan monitors. [3]
The same study found the black-palmed rock monitor, the largest species within Odatria, as sister to the rest of Odatria, making it the most basal species within the subgenus. [3] Vidal et al. 2012 also recovers this species as the most basal, although within the V. tristis group. [4]
During the early-mid Oligocene, the genus Varanus dispersed in two directions. The southeastern dispersion into Indo-Australia likely occurred shortly after the collision of the Asian and Australian tectonic plates, which created a connection between Sahul and Sundaland, i.e., an Indonesian land bridge that would have facilitated the dispersal of monitor lizards into Australia. The existence of this land bridge also likely allowed the Indo-Australopapuan clade including many Odatria species to disperse back into Indonesia. [3]
Phylogeny
Species Complexes
Within scientific literature, the V. acanthurus complex has included V. acanthurus, V. baritji, V. primordius, and V. storri, while the V. timorensis complex has included V. auffenbergi, V. scalaris, V. similis, V. timorensis, and V. tristis. In light of phylogenetic analysis by Vidal et al. 2012 and Brennan et al. 2020 however, to maintain monophyly, more species would be included in these two species complexes, as in the following:
V. acanthurus complex - (V. acanthurus, V. baritji, V. kingorum, V. primordius, and V. storri)
V. timorensis complex - (V. auffenbergi, V. glauerti, V. mitchelli, V. scalaris, V. semiremex, V. similis, V. timorensis, and V. tristis)
Species Groups
Vidal et al. 2012 separates Odatria into two species groups; the V. acanthurus group includes V. acanthurus, V. baritji, V. brevicauda, V. bushi, V. caudolineatus, V. eremius, V. gilleni, V. kingorum, V. primordius, and V. storri, while the V. tristis group includes V. auffenbergi, V. glauerti, V. glebopalma, V. mitchelli, V. pilbarensis, V. scalaris, V. semiremex, V. similis, V. timorensis, and V. tristis. [4]
To maintain monophyly in light of Brennan et al. 2020, V. glebopalma would be excluded from the V. tristis group, and more species would be included as in the following:
V. acanthurus group - (V. acanthurus, V. baritji, V. brevicauda, V. bushi, V. caudolineatus, V. eremius, V. gilleni, V. kingorum, V. primordius, V. sparnus and V. storri)
V. tristis group - (V. auffenbergi, V. glauerti, V. hamersleyensis, V. mitchelli, V. pilbarensis, V. scalaris, V. semiremex, V. similis, V. timorensis, and V. tristis)
| Brennan et al. 2020 | Vidal et al. 2012 |
|---|---|
 |
.png.webp) |
Ecology
Behaviour
The genus is diverse in terms of behaviour, and includes terrestrial, arboreal, and semiaquatic species. Many are saxicolous, i.e., they are strongly associated with rocky habitats, while some others prefer forests. [2]
Ritualized fighting sometimes occurs between males. Unlike many larger monitors which grapple with each other while standing on their hind legs, some dwarf monitor species instead grapple each other with all 4 limbs, belly to belly, and roll around on the ground trying to force the other onto its back. Biting may also occur.[5]
Diet
Orthopteran insects and other species of lizards make up the mainstay of the diet for most species. One species, the rusty desert monitor, consumes a particularly large amount of lizard prey with lizards such as various species of Ctenotus accounting for up to 70% of their diet by weight. [6] Larger species such as black-palmed rock monitors are also known to prey on other dwarf monitors. [2]
Reproduction
Like all other monitors, all species within Odatria are oviparous. As parthenogenesis is well known in monitor lizards in some other subgenera, it is possible that dwarf monitors are also capable of parthenogenesis; there is a report of a suspected case of parthenogenesis in peacock monitors. [2]
Parasites
Physalopterid nematodes of the genus Abbreviata are major gastrointestinal parasites in many species of reptiles. Those that parasitize dwarf monitors include A. hastaspicula and A. levicauda; the former may specialize in Australian monitors. Depending on the species, more than half of all individuals collected from the wild may be infected, but in most cases the parasites do minimal harm. Various other parasites are also known, but in comparatively much low numbers. [1][7] In captivity, wild caught individuals may suffer more from the effects of parasites due to the stressful process of being taken from the wild and being shipped elsewhere.
Conservation and captivity
Under the EPBC Act, the export of live Australian Odatria species for commercial purposes is prohibited. Illegal trade of these species still persist, as many dwarf monitor species are in great demand in the pet trade. In Australia, monitor lizards are threatened by the invasive cane toad, as they have no natural immunity to the toad's toxins when ingested. [2]
Captive breeding for the pet trade has been successful in many such species however and lessens the pressure on wild populations. Some such as the often captive bred ridge-tailed monitor are popular pet lizards, in some cases as a "beginner monitor" to train those who are aspiring to eventually keep larger and less forgiving monitor lizard species.
Species
| Species | Taxon author | Common name | Picture |
|---|---|---|---|
| V. glebopalma | Mitchell, 1955 | Black-palmed rock monitor |
| Species | Taxon author | Common name | Picture |
|---|---|---|---|
| V. acanthurus | Boulenger, 1885 | Ridge-tailed monitor |  |
| V. baritji | King & Horner, 1987 | White's dwarf monitor | |
| V. brevicauda | Boulenger, 1898 | Short-tailed monitor | |
| V. bushi | Applin et al., 2006 | Pilbara stripe-tailed monitor | |
| V. caudolineatus | Boulenger, 1885 | Stripe-tailed monitor | .jpg.webp) |
| V. eremius | Lucas & Frost, 1895 | Rusty desert monitor | |
| V. gilleni | Lucas & Frost, 1895 | Pygmy mulga monitor |  |
| V. kingorum | Storr, 1980 | King's rock monitor |  |
| V. primordius | Mertens, 1942 | Northern ridge-tailed monitor | |
| V. sparnus | Doughty et al., 2014 | Dampier Peninsula monitor | |
| V. storri | Mertens, 1966 | Storr's monitor |  |
| Species | Taxon author | Common name | Picture |
|---|---|---|---|
| V. auffenbergi | Sprackland, 1999 | Peacock monitor |  |
| V. glauerti | Mertens, 1957 | Kimberley rock monitor | .jpg.webp) |
| V. hamersleyensis | Maryan et al., 2014 | Hamersley Range rock monitor |  |
| V. mitchelli | Mertens, 1958 | Mitchell's water monitor |  |
| V. pilbarensis | Storr, 1980 | Pilbara rock monitor | |
| V. scalaris | Mertens, 1941 | Banded tree monitor | |
| V. semiremex | Peters, 1869 | Rusty monitor | |
| V. similis | Mertens, 1958 | Spotted tree monitor | |
| V. timorensis | Gray, 1831 | Timor monitor |  |
| V. tristis | Schlegel, 1838 | Black headed monitor
Freckled monitor |
 |
References
- Jones, Hugh I. (2010-07-28). "Dwarf monitor lizards (Varanidae : Varanus, Odatria s. gen.) as definitive and paratenic hosts for physalopteran nematodes". Australian Journal of Zoology. 58 (2): 69–75. doi:10.1071/ZO09122. ISSN 1446-5698.
- Auliya, Mark (2020). Visual Identification Guide for the Monitor Lizard Species of the World (Genus Varanus). Bonn, Germany: Bundesamt für Naturschutz (BfN). pp. 80–122. ISBN 978-3-89624-290-7.
- Brennan, Ian G.; Lemmon, Alan R.; Lemmon, Emily Moriarty; Portik, Daniel M.; Weijola, Valter; Welton, Luke; Donnellan, Stephen C.; Keogh, J. Scott (2020-02-03). "Phylogenomics of monitor lizards and the role of competition in dictating body size disparity". bioRxiv: 2020.02.02.931188. doi:10.1101/2020.02.02.931188. PMID 32521014. S2CID 211297088.
- Vidal, Nicolas; Marin, Julie; Sassi, Julia; Battistuzzi, Fabia U.; Donnellan, Steve; Fitch, Alison J.; Fry, Bryan G.; Vonk, Freek J.; Rodriguez de la Vega, Ricardo C.; Couloux, Arnaud; Hedges, S. Blair (2012-10-23). "Molecular evidence for an Asian origin of monitor lizards followed by Tertiary dispersals to Africa and Australasia". Biology Letters. 8 (5): 853–855. doi:10.1098/rsbl.2012.0460. ISSN 1744-9561. PMC 3441001. PMID 22809723.
- Bennett, Daniel (1995). A little book of monitor lizards. Viper Press.
- Jones, Hugh I. (2010). "Dwarf monitor lizards (Varanidae:Varanus, Odatria s. gen.) as definitive and paratenic hosts for physalopteran nematodes". Australian Journal of Zoology. 58 (2): 69. doi:10.1071/ZO09122. ISSN 0004-959X.
- King, C.; Jones, H.I. (December 2016). "The life cycle of the reptile-inhabiting nematode Abbreviata hastaspicula (Spirurida: Physalopteridae: Physalopterinae) in Australia". International Journal for Parasitology: Parasites and Wildlife. 5 (3): 258–262. doi:10.1016/j.ijppaw.2016.08.002. PMC 5024145. PMID 27668177.
_(9334711410).jpg.webp)