Universal flu vaccine
A universal flu vaccine is a flu vaccine that is effective against all influenza strains regardless of the virus sub type, antigenic drift or antigenic shift.[1][2][3] Hence it should not require modification from year to year. As of 2019 there has been no approved universal flu vaccine for general use, but several have been in development.[1]
Medical uses
Influenza vaccines are required every year due to the diversity of flu viruses and variable efficacy of vaccines to prevent them. By creating a universal vaccine the need to recreate a yearly shot is eliminated. The efficacy of a vaccine refers to the protection among a broad variety of influenza strains. Events such as antigenic shift have created pandemic strains such as the H1N1 outbreak in 2009. The research required every year to isolate a potential popular viral strain and create a vaccine to defend against it is a six month long process. During that time the virus can mutate making the vaccines less effective.[4]
High risk populations including the elderly and those with chronic disease often have limited immunity towards the flu from vaccines. The vaccines have been proven to be 30% to 70% effective in preventing hospitalization from the flu or pneumonia.
On average influenza vaccine efficacy is 60% among the general population that receive yearly vaccinations.
A universal vaccine could be reproduced at high levels and eliminate availability and supply issues of current vaccines.[5] There is conflicting evidence on whether it would cut costs.[6]
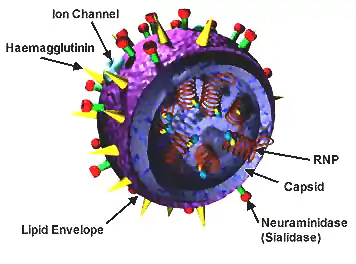
Structure of influenza
Influenza A is involved in most strains of the flu. It is an enveloped RNA virus. It has a protein membrane containing the glycoproteins hemagglutinin (HA) and neuraminidase (NA) which are used by the virus to enter a host cell and to release itself and its copies from the host cell. Each strain of the influenza virus has a different pattern of glycoproteins; the glycoproteins themselves have variability as well.[7]
History
.png.webp)
In 2008, Acambis announced work on a universal flu vaccine (ACAM-FLU-ATM) based on the less variable M2 protein component of the flu virus shell.[8] See also H5N1 vaccines.
In 2009, the Wistar Institute in Pennsylvania received a patent for using "a variety of peptides" in a flu vaccine, and announced it was seeking a corporate partner.[9]
In 2010, the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. NIH announced a breakthrough; the effort targets the stem, which mutates less often than the head of the viral HA.[10]
By 2010 some universal flu vaccines had started clinical trials.
- BiondVax identified 9 conserved epitopes of the influenza virus and combined them into a recombinant protein called Multimeric-001.[11][12] To date (August 2020), all seven of Biondvax's completed phase 2 human trials have demonstrated safety and significant levels of immunogenicity. More recently, Biondvax (NASDAQ:BVXV) has completed the second year of a two year, more than 12,400 participant pivotal phase 3 study of Multimeric-001, its candidate universal influenza vaccine. Biondvax expects results of its phase 3 trial between end of September and end of October 2020. Should it be successful, as have all seven previous phase 2 trials, it will be brought to market early next year (2021). Production of the Multimeric-001 is at their manufacturing plant in Jerusalem.
- ITS's fp01[13] includes 6 peptide antigens to highly conserved segments of the PA, PB1, PB2, NP & M1 proteins, and has started phase I trials.
DNA vaccines, such as VGX-3400X (aimed at multiple H5N1 strains), contain DNA fragments (plasmids).[14][15] Inovio's SynCon DNA vaccines include H5N1 and H1N1 subtypes.[16]
Other companies pursuing the vaccine as of 2009 and 2010 include Theraclone,[17] VaxInnate,[18] Crucell NV,[19] Inovio Pharmaceuticals,[14] Immune Targeting Systems (ITS)[20] and iQur.[21]
In 2019, Distributed Bio completed pre-clinical trials of a vaccine that consists of computationally selected distant evolutionary variants of hemagglutinin epitopes and is expected to begin human trials in 2021.[22]
In recent years, research has concerned use of an antigen for the flu hemagglutinin (HA) stem. Based on the results of animal studies, a universal flu vaccine may use a two-step vaccination strategy: priming with a DNA-based HA vaccine, followed by a second dose with an inactivated, attenuated, or adenovirus-vector-based vaccine.[23]
Some people given a 2009 H1N1 flu vaccine have developed broadly protective antibodies, raising hopes for a universal flu vaccine.[24][25][26]
A 'vaccine'/antigen based on the hemagglutinin (HA) stem was the first to induce 'broadly neutralizing' antibodies to both HA-group 1 and HA-group 2 influenza in mice.[27]
In July 2011, researchers created an antibody, which targets a protein found on the surface of all influenza A viruses called haemagglutinin.[28][29][30] FI6 is the only known antibody that binds (its neutralizing activity is controversial) to all 16 subtypes of the influenza A virus hemagglutinin and might be the lynchpin for a universal influenza vaccine.[28][29][30] The subdomain of the hemagglutinin that is targeted by FI6, namely the stalk domain, was actually successfully used earlier as universal influenza virus vaccine by Peter Palese's research group at Mount Sinai School of Medicine.[31]
Other vaccines are polypeptide based.[32]
Research
A study from the Albert Einstein College of Medicine, where researchers deleted gD-2 from the herpes virus, which is responsible for HSV microbes entering in and out of cells showed as of May 1st, 2018 the same vaccine can be used in a modified way to contain hemagglutinin and invoke a special ADCC immune response.[33]
The Washington University School of Medicine in St.Louis and the Icahn School of Medicine in Mount Sinai in New York are using the glycoprotein neuraminidase as a targeted antigen in their research. Three monoclonal antibodies (mAB) were sampled from a patient infected with influenza A H3N2 virus. The antibodies were able to bind to the neuraminidase active site neutralizing the virus across multiple strains. The site remains the same with minimal variability across most of the flu strains. In trials using mice all three antibodies were effective across multiple strains, one antibody was able to protect the mice from all 12 strains tested including human and non-human flu viruses. All mice used in the experiments survived even if the antibody was not administered until 72 hours after the time of infection.[34]
Simultaneously the NIAID is working on a peptide vaccine that is starting human clinical trials in the 2019 flu season. The study will include 10,000 participants who will be monitored for two flu seasons. The vaccine will show efficacy if it is able to reduce the number of influenza cases in all strains.[35]
In 2019-2020, a vaccine candidate from Peter Palese's group at Mount Sinai Hospital emerged from a phase 1 clinical trial with positive results. By vaccinating twice with hemagglutinins that have different "heads" but the same membrane-proximal "stalk", the immune system is directed to focus its attention on the conserved stalk. [36][37]
References
- Nachbagauer R, Krammer F (April 2017). "Universal influenza virus vaccines and therapeutic antibodies". Clinical Microbiology and Infection. 23 (4): 222–228. doi:10.1016/j.cmi.2017.02.009. PMC 5389886. PMID 28216325.
- Khanna, Madhu; Sharma, Sachin; Kumar, Binod; Rajput, Roopali (2014). "Protective Immunity Based on the Conserved Hemagglutinin Stalk Domain and Its Prospects for Universal Influenza Vaccine Development". BioMed Research International. 2014: 546274. doi:10.1155/2014/546274. ISSN 2314-6133. PMC 4055638. PMID 24982895.
- Sherwood Linda M, Prescott's Microbiology (10th ed.) McGraw-Hill Education , 2017
- Corona A. “A Universal Influenza Vaccine: How Close Are we?” "American Society for Microbiology", 2019
- Ben-Yedidia T and Gottlieb T, “Epitope-based approaches to a universal influenza vaccine” "Journal of Autoimmunity" , 2014
- France G, "Poteintial cost effecitiveness of a universal vaccine in older adults" "ncbi" , 2018
- Sherwood Linda M, Prescott's Microbiology (10th ed.) McGraw-Hill Education , 2017
- "Universal Influenza Vaccine Tested Successfully In Humans". ScienceDaily.
- The Wistar Institute obtains patent for universal flu vaccine technology Archived January 10, 2010, at the Wayback Machine. Wistar Institute.
- NIH Scientists Advance Universal Flu Vaccine. NIH.
- Shpurer, Sharon (22 April 2007). "Copaxone Inventor Ruth Arnon Taking BiondVax Public". Haaretz.
- Dance, Amber (30 March 2012). "News: Moving towards a universal flu vaccine". Nature. doi:10.1038/nature.2012.10333.
- Immune Targeting Systems – FP01 Influenza, undated page Archived January 31, 2015, at the Wayback Machine
- "Inovio Pharmaceuticals, Inc. Immunizes First Subject In U.S. Influenza DNA Vaccine Clinical Trial". Reuters.
- "Inovio Biomedical's SynCon preventive DNA vaccine receives approval in Korea for Phase I clinical trial". News-Medical.net. March 2, 2010.
- "Scientific Paper on Inovio Pharmaceuticals SynCon(TM) DNA Vaccines and Intradermal DNA Delivery Technology One of Most Cited Articles in the Journal Vaccine". October 14, 2010.
- Seattle's Theraclone makes a 'first step' on long road to universal flu vaccine Archived November 16, 2011, at the Wayback Machine. The Seattle Times.
- VaxInnate's Universal Flu Vaccine Candidate Shown Safe and Immunogenic in Phase I Clinical Study. Fierce Biotech.
- Johnson & Johnson pursues vaccine firm. Charleston Gazette.
- Immune Targeting Systems – About Us Archived January 27, 2015, at the Wayback Machine
- "FLUTCORE Report Summary". European Commission. Archived from the original on 27 April 2017.
- "Centivax Universal Influenza Vaccine | Grand Challenges". gcgh.grandchallenges.org. Retrieved 2020-01-28.
- Lambert LC, Fauci AS (November 2010). "Influenza vaccines for the future". The New England Journal of Medicine. 363 (21): 2036–44. doi:10.1056/NEJMra1002842. PMID 21083388.
- "H1N1 Gives Clues to Universal Flu Vaccine". January 18, 2011.
- Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, et al. (January 2011). "Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection". The Journal of Experimental Medicine. 208 (1): 181–93. doi:10.1084/jem.20101352. PMC 3023136. PMID 21220454.
- "A vaccine for all flu seasons". Spring 2011.
- Stalking influenza by vaccination with pre-fusion headless HA mini-stem. 2016
- BBC: 'Super antibody' fights off flu
- "Scientists hail the prospect of a universal vaccine for flu". The Independent. July 29, 2011.
- "Universal Flu Vaccine On The Horizon: Researchers Find 'Super Antibody'" The Huffington Post. July 28, 2011
- Influenza Virus Vaccine Based on the Conserved Hemagglutinin Stalk Domain
- Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, García-Sastre A, Moran TM, Palese P (November 2010). "Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes". Proceedings of the National Academy of Sciences of the United States of America. 107 (44): 18979–84. Bibcode:2010PNAS..10718979W. doi:10.1073/pnas.1013387107. PMC 2973924. PMID 20956293.
- "The Second Annual Einstein-Montefiore Presidential Lecture: William R. Jacobs, Ph.D., Lecture (3of3)" – via www.youtube.com.
- Stadlbauer D, “Broadly protective human antibodies that target the active site of influenza virus neuraminidase” "Science", 2019
- Taylor A, "First Universal Flu Vaccine to Enter Phase 3 Trial" "The Scientist", 2018
- Bernstein, David I; Guptill, Jeffrey; Naficy, Abdollah; Nachbagauer, Raffael; Berlanda-Scorza, Francesco; Feser, Jodi; Wilson, Patrick C; Solórzano, Alicia; Van der Wielen, Marie; Walter, Emmanuel B; Albrecht, Randy A; Buschle, Kristen N; Chen, Yao-qing; Claeys, Carine; Dickey, Michelle; Dugan, Haley L; Ermler, Megan E; Freeman, Debra; Gao, Min; Gast, Christopher; Guthmiller, Jenna J; Hai, Rong; Henry, Carole; Lan, Linda Yu-Ling; McNeal, Monica; Palm, Anna-Karin E; Shaw, Dustin G; Stamper, Christopher T; Sun, Weina; Sutton, Victoria; Tepora, Micah E; Wahid, Rahnuma; Wenzel, Heather; Wohlbold, Teddy John; Innis, Bruce L; García-Sastre, Adolfo; Palese, Peter; Krammer, Florian (January 2020). "Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial". The Lancet Infectious Diseases. 20 (1): 80–91. doi:10.1016/S1473-3099(19)30393-7. PMID 31630990.
- Nachbagauer, Raffael; Feser, Jodi; Naficy, Abdollah; Bernstein, David I.; Guptill, Jeffrey; Walter, Emmanuel B.; Berlanda-Scorza, Franceso; Stadlbauer, Daniel; Wilson, Patrick C.; Aydillo, Teresa; Behzadi, Mohammad Amin; Bhavsar, Disha; Bliss, Carly; Capuano, Christina; Carreño, Juan Manuel; Chromikova, Veronika; Claeys, Carine; Coughlan, Lynda; Freyn, Alec W.; Gast, Christopher; Javier, Andres; Jiang, Kaijun; Mariottini, Chiara; McMahon, Meagan; McNeal, Monica; Solórzano, Alicia; Strohmeier, Shirin; Sun, Weina; Van der Wielen, Marie; Innis, Bruce L.; García-Sastre, Adolfo; Palese, Peter; Krammer, Florian (7 December 2020). "A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial". Nature Medicine: 1–9. doi:10.1038/s41591-020-1118-7.
Further reading
- Sautto GA, Kirchenbaum GA, Ross TM (January 2018). "Towards a universal influenza vaccine: different approaches for one goal". Virology Journal. 15 (1): 17. doi:10.1186/s12985-017-0918-y. PMC 5785881. PMID 29370862.
- Sano K, Ainai A, Suzuki T, Hasegawa H (September 2017). "The road to a more effective influenza vaccine: Up to date studies and future prospects". Vaccine. 35 (40): 5388–5395. doi:10.1016/j.vaccine.2017.08.034. PMID 28866292.
- Krammer F (May 2017). "Strategies to induce broadly protective antibody responses to viral glycoproteins". Expert Review of Vaccines. 16 (5): 503–513. doi:10.1080/14760584.2017.1299576. PMID 28277797.
- Stadlbauer D, Nachbagauer R, Meade P, Krammer F (December 2017). "Universal influenza virus vaccines: what can we learn from the human immune response following exposure to H7 subtype viruses?". Frontiers of Medicine. 11 (4): 471–479. doi:10.1007/s11684-017-0602-z. PMID 29159597.