Tetrapropylammonium perruthenate
Tetrapropylammonium perruthenate (TPAP or TPAPR) is the chemical compound described by the formula N(C3H7)4RuO4. Sometimes known as the Ley–Griffith reagent, this ruthenium compound is used as a reagent in organic synthesis. This salt consists of the tetrapropylammonium cation and the perruthenate anion, RuO−
4.
 | |
| Names | |
|---|---|
| IUPAC name
Tetrapropylammonium perruthenate | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | TPAP TPAPR |
| ChemSpider | |
| ECHA InfoCard | 100.156.687 |
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H28NRuO4 | |
| Molar mass | 351.43 g/mol |
| Appearance | Green solid |
| Melting point | 160 °C (320 °F; 433 K) (decomposition) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Ruthenium tetroxide is a highly aggressive oxidant, but TPAP, which is its one-electron reduced derivative, is a mild oxidizing agent for the conversion of primary alcohols to aldehydes (the Ley oxidation).[1] Secondary alcohols are similarly oxidized to ketones.[2] It can also be used to oxidize primary alcohols all the way to the carboxylic acid with a higher catalyst loading, larger amount of the co-oxidant, and addition of two equivalents of water. In this situation, the aldehyde reacts with water to form the geminal-diol hydrate, which is then oxidized again.[3]
The oxidation generates water that can be removed by adding molecular sieves. TPAP is expensive, but it can be used in catalytic amounts. The catalytic cycle is maintained by adding a stoichiometric amount of a co-oxidant such as N-methylmorpholine N-oxide[4] or molecular oxygen.[5]
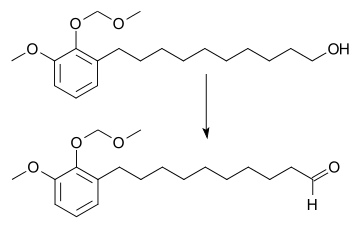
TPAP is also used to cleave vicinal diols to form aldehydes.[2]
References
- Ley, Steven V.; Norman, Joanne; Griffith, William P.; Marsden, Stephen P. (1994). "Tetrapropylammonium perruthenate, Pr4N+RuO4−, TPAP: A catalytic oxidant for organic synthesis". Synthesis. 1994 (7): 639–666. doi:10.1055/s-1994-25538. (review article)
- Ley, Steven V.; Norman, Joanne; Wilson, Anthony J. (2011), "Tetra-n-propylammonium Perruthenate", Encyclopedia of Reagents for Organic Synthesis, Chichester, UK: John Wiley & Sons, Ltd, pp. rt074.pub2, doi:10.1002/047084289x.rt074.pub2, ISBN 978-0-471-93623-7, retrieved 2020-09-04
- Xu, Z.; Johannes, C. W.; Houri, A. F.; La, D. S.; Cogan, D. A.; Hofilena, G. E.; Hoveyda, A. H. (1997). "Applications of Zr-catalyzed carbomagnesation and Mo-catalyzed macrocyclic ring closing metathesis in asymmetric synthesis. Enantioselective total synthesis of Sch 38516 (Fluvirucin B1)". J. Am. Chem. Soc. 119 (43): 10302–10316. doi:10.1021/ja972191k.
- Griffith, William P.; Ley, Steven V.; Whitcombe, Gwynne P.; White, Andrew D. (1987). "Preparation and use of tetra-n-butylammonium per-ruthenate (TBAP reagent) and tetra-n-propylammonium per-ruthenate (TPAP reagent) as new catalytic oxidants for alcohols". J. Chem. Soc., Chem. Commun. (21): 1625–1627. doi:10.1039/C39870001625.
- Lenz, Roman; Ley, Steven V. (1997). "Tetra-n-propylammonium perruthenate (TPAP)-catalysed oxidations of alcohols using molecular oxygen as a co-oxidant". J. Chem. Soc., Perkin Trans. 1 (22): 3291–3292. doi:10.1039/A707339I.
- Hadfield, John A.; McGown, Alan T.; Butler, John (2000). "A high-yielding synthesis of the naturally occurring antitumour agent irisquinone" (PDF). Molecules. 5 (12): 82–88. doi:10.3390/50100082.