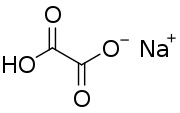
Sodium hydrogenoxalate
Sodium hydrogenoxalate is the sodium salt of hydrogenoxalate. The only difference from oxalic acid is that one of the two hydrogen atoms has been replaced with a sodium atom. Like oxalate, it is toxic for the kidney function if swallowed because of the precipitation of poorly soluble calcium oxalate stones that can obstruct the kidney tubules.[6]
 | |
| Names | |
|---|---|
| IUPAC name
Sodium hydrogen oxalate[1] | |
| Other names
Ethanedioate, hydrogen sodium salt (1:1:1) Ethanedioic acid, sodium salt (1:1) Monosodium oxalate Sodium hydrogen ethanedioate (1:1:1) Oxalic acid sodium salt Oxalic acid, sodium salt Sodium acid oxalate Sodium and hydrogen and oxalate Sodium bioxalate[2] | |
| Identifiers[3][4] | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.356 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2HNaO4 | |
| Molar mass | 112.0167[5] |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H302, H312 | |
| P264, P270, P280, P301+312, P302+352, P312, P322, P330, P363, P501 | |
| Related compounds | |
Other anions |
Sodium bicarbonate (oxalate replaced with carbonate) |
Other cations |
Potassium hydrogenoxalate (potassium instead of sodium) Oxalic acid (hydrogen instead of sodium) Sodium oxalate (sodium instead of hydrogen) |
Related compounds |
Hydrogenoxalate (sodium ion removed) Hydrogenoxalate (sodium and hydrogen ions removed) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Stability
Upon being heated, sodium hydrogenoxalate undergoes cation-pairing to become oxalic acid and sodium oxalate, the latter of which decomposes into sodium carbonate and carbon monoxide.
References
- "2T9TH558WS | C2HNaO4 | ChemSpider". www.chemspider.com. p. Names. Retrieved 11 September 2018.
Sodium hydrogen oxalate [ACD/IUPAC Name]
- "2T9TH558WS | NaHC2O4 | ChemSpider". www.chemspider.com. p. Names. Retrieved 11 September 2018.
- "Monosodium oxalate". pubchem.ncbi.nlm.nih.gov. Retrieved 11 September 2018.
- "2T9TH558WS | NaHC2O4 | ChemSpider". www.chemspider.com. p. Names. Retrieved 11 September 2018.
- "Sodium Hydrogen Oxalate NaHC2O4 Molecular Weight — EndMemo". www.endmemo.com. Retrieved 11 September 2018.
Molar Mass: 112.0167
- "Monosodium oxalate". pubchem.ncbi.nlm.nih.gov. Retrieved 11 September 2018.
H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (100%): Harmful in contact with skin [Warning Acute toxicity, dermal]
- W. Balcerowiak; Cz. Latocha; J. Wasilewski (1980). "Thermoanalytical investigation of mixtures containing oxalic acid, sodium hydrogen oxalate and sodium oxalate". Journal of Thermal Analysis. 18: 57–63. doi:10.1007/BF01909453.
A solid ternary system containing oxalic acid dihydrate, sodium hydrogen oxalate was subjected to thermoanalytical investigation to develop its full qualitative and quantitative analyses based on the following reactions of thermal decomposition of the individually heated compounds of this system [4-6]: H2C2O4 " 2 H2O -+ H2C2O4 + 2 H2O NaHC2O4 9 H2O -* NaHC2O4 + H2O 2 NaHC2O4 ~ Na2C2O4 + (H2C2O4) Na2C2O4 --, Na2CO3 + CO
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.