Hydrogenoxalate
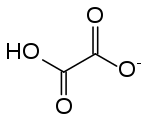
Hydrogenoxalate or hydrogen oxalate is an anion with chemical formula HC
2O−
4 or HO
2C–CO−
2, derived from oxalic acid by the loss of a single proton; or, alternatively, from the oxalate anion C
2O2−
4 by addition of a proton.
 | |
| Names | |
|---|---|
| IUPAC name
Hydrogen ethanedioate | |
| Other names
Hydrogen oxalate 2-Hydroxy-2-oxoacetate | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C2HO4− | |
| Molar mass | 89.027 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The name is also used for any salt containing this anion, such as NaHC2O4, KHC2O4, or NH3HC2O4. Hydrogenoxalates may also be called (especially in older literature) bioxalates, acid oxalates, or monobasic oxalates.
See also
References
- Tellgren, Roland; Olovsson, Ivar (1971). "The crystal structures of normal and deuterated sodium hydrogen oxalate monohydrate NaHC2O4·H2O and NaDC2O4·D2O. Hydrogen bond studies XXXVI". The Journal of Chemical Physics. 54: 127–134. Bibcode:1971JChPh..54..127T. doi:10.1063/1.1674582.
- Delaplane, R. G.; Tellgren, R.; Olovsson, I. (1984). "Neutron diffraction study of sodium hydrogen oxalate monohydrate, NaHC2O4·H2O, at 120 K". Acta Crystallographica. C40: 1800–1803. doi:10.1107/S0108270184009616.
- Hamadène, M.; Kherfi, H.; Guehria-Laidoudi, A. (2006). "The polymeric anhydrous rubidium hydrogen oxalate". Acta Crystallographica. A62: s280.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.