Segesterone
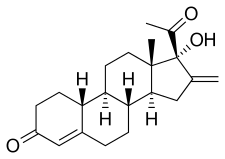
Segesterone (INN, USAN),[1][2] also known as 17α-hydroxy-16-methylene-19-norprogesterone or as 17α-deacetylnestorone, is a steroidal progestin of the 19-norprogesterone group that was never marketed.[3] An acetate ester, segesterone acetate, better known as nestorone or elcometrine, is marketed for clinical use.[4] Segesterone acetate produces segesterone as a metabolite.[5]
 | |
| Clinical data | |
|---|---|
| Other names | 17α-Hydroxy-16-methylene-19-norprogesterone; 16-Methylene-17α-hydroxy-19-norpregn-4-ene-3,20-dione; 17α-Deacetylnestorone |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H28O3 |
| Molar mass | 328.452 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- https://www.who.int/medicines/publications/druginformation/innlists/PL89.pdf
- http://chem.sis.nlm.nih.gov/chemidplus/rn/7690-08-6
- https://pubchem.ncbi.nlm.nih.gov/compound/11823650
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1403–. ISBN 978-1-60913-345-0.
- Prasad PV, Bashir M, Sitruk-Ware R, Kumar N (2010). "Single-dose pharmacokinetics of Nestorone, a potential female-contraceptive". Steroids. 75 (3): 252–64. doi:10.1016/j.steroids.2009.12.011. PMID 20064539. S2CID 205253216.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.