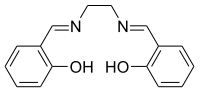
Salen ligand
Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states.[1] These metal salen complexes primarily find use as catalysts.[2]
 | |
| Names | |
|---|---|
| Other names
2,2′-Ethylenebis(nitrilomethylidene)diphenol, N,N′-Ethylenebis(salicylimine) | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.161 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H16N2O2 | |
| Molar mass | 268.32 |
| Melting point | 126 °C (259 °F; 399 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and properties
H2salen may be synthesized by the condensation of ethylenediamine and salicylaldehyde.[3]
.png.webp)
Complexes of salen with metal cations may be made without isolating it from the reaction mixture.[4][5] This is possible because the stability constant for the formation of the metal complexes are very high, due to the chelate effect.
- H2L + Mn+ → ML(n−2)+ + 2 H+
where L stands for the ligand. The pyridine adduct of the cobalt(II) complex Co(salen)(py) (salcomine) has a square-pyramidal structure; it can act as a dioxygen carrier by forming a labile, octahedral O2 complex.[6][7]
The name “salen ligands” is used for tetradentate ligands which have similar structures. For example, in salpn there is a methyl substituent on the bridge. It is used as a metal deactivation additive in fuels.[8] The presence of bulky groups near the coordination site may enhance the catalytic activity of a metal complex and prevent its dimerization. Salen ligands derived from 3,5-di-tert-butylsalicylaldehyde fulfill these roles, and also increase the solubility of the complexes in non-polar solvents like pentane. Chiral “salen” ligands may be created by proper substitution of the diamine backbone, the phenyl ring, or both.[5] An example is the ligand obtained by condensation of the C2-symmetric trans-1,2-diaminocyclohexane with 3,5-di-tert-butylsalicylaldehyde. Chiral ligands may be used in asymmetric synthesis reactions, such as the Jacobsen epoxidation:[9][10]
Related ligands
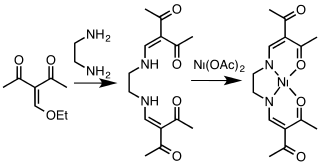
A class of tetradentate ligands with the generic name acacen are obtained by the condensation of derivatives of acetylacetone and ethylenediamine.[11] Cobalt complexes [Co(acacen)L2]+, selectively inhibit the activities of histidine-containing proteins through exchange of the axial ligands. These compounds show promise for the inhibition of oncogenesis.[12]
The salan and salalen ligands are similar in structure to salen ligands, but have one or two saturated nitrogen-aryl bonds (amines rather than imines). They tend to be less rigid and more electron rich at the metal center than the corresponding salen complexes.[13][14] Salans can be synthesized by the alkylation of an appropriate amine with a phenolic alkyl halide. The “half-salen” ligands have only one salicylimine group. They are prepared from a salicylaldehyde and a monoamine.[15]
The name “salen” or “salen-type” may be used for other ligands that have similar environment around the chelating site, namely two acidic hydroxyls and two Schiff base (aryl-imine) groups. These include the ligands abbreviated as salph, from the condensation of 1,2-phenylenediamine and salicylaldehyde, and salqu, from the condensation of salicylaldehyde and 2-quinoxalinol.[16]
See also
- Metal salen complexes
- Bisthiosemicarbazones, a structurally related class of C2-symmetric imine based ligands
References
- Cozzi, Pier Giorgio (2004). "Metal–Salen Schiff base complexes in catalysis: practical aspects". Chem. Soc. Rev. 33 (7): 410–421. doi:10.1039/B307853C. PMID 15354222.
- Shaw, Subrata; White, James D. (11 June 2019). "Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances". Chemical Reviews. 119 (16): 9381–9426. doi:10.1021/acs.chemrev.9b00074. PMID 31184109.
- Tsumaki, T. (1938). "Nebenvalenzringverbindungen. IV. Über einige innerkomplexe Kobaltsalze der Oxyaldimine". Bulletin of the Chemical Society of Japan (in German). 13 (2): 252–260. doi:10.1246/bcsj.13.252.
- Diehl, Harvey; Hach, Clifford C. (1950). Bis(N,N′-Disalicylalethylenediamine)-μ-Aquodicobalt(II). Inorg. Synth. Inorganic Syntheses. 3. pp. 196–201. doi:10.1002/9780470132340.ch53. ISBN 978-0-470-13234-0.
- Pier Giorgio Cozzi (2004). "Metal-Salen Schiff base complexes in catalysis: Practical aspects". Chem. Soc. Rev. 33 (7): 410–421. doi:10.1039/B307853C. PMID 15354222.
- Appleton, T. G. (1977). "Oxygen Uptake by a Cobalt(II) Complex". J. Chem. Educ. 54 (7): 443. doi:10.1021/ed054p443.
- Yamada, Shoichiro (1999). "Advancement in stereochemical aspects of Schiff base metal complexes". Coordination Chemistry Reviews. 190–192: 537–555. doi:10.1016/S0010-8545(99)00099-5.
- Dabelstein, W.; Reglitzky A.; Schutze A.; Reders, K. "Automotive Fuels". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_719.pub2.CS1 maint: multiple names: authors list (link)
- Larrow, J. F.; Jacobsen, E. N. (2004). "(R,R)-N,N'-Bis(3,5-Di-tert-Butylsalicylidene)-1,2-Cyclohexanediamino Manganese(III) Chloride, A Highly Enantioselective Epoxidation Catalyst". Organic Syntheses.; Collective Volume, 10, p. 96
- Yoon, TP; Jacobsen, EN (2003). "Privileged Chiral Catalysts". Science. 299 (5613): 1691–1693. Bibcode:2003Sci...299.1691Y. doi:10.1126/science.1083622. PMID 12637734. S2CID 27416160.
- Weber, Birgit; Jäger, Ernst-G. (2009). "Structure and Magnetic Properties of Iron(II/III) Complexes with N
2O2–
2-Coordinating Schiff Base-Like Ligands". Eur. J. Inorg. Chem.: 455. doi:10.1002/ejic.200990003. - Bajema, Elizabeth A.; Kaleigh F. Roberts; Meade, Thomas J. (2019). "Chapter 11. Cobalt-Schiff Base Complexes:Preclinical Research and Potential Therapeutic Uses". In Sigel, Astrid; Freisinger, Eva; Sigel, Roland K. O.; Carver, Peggy L. (Guest editor) (eds.). Essential Metals in Medicine:Therapeutic Use and Toxicity of Metal Ions in the Clinic. Metal Ions in Life Sciences. 19. Berlin: de Gruyter GmbH. pp. 267–301. doi:10.1515/9783110527872-017. ISBN 978-3-11-052691-2. PMID 30855112.
- Atwood, David A.; Remington, Michael P.; Rutherford, Drew (1996). "Use of the Salan Ligands to Form Bimetallic Aluminum Complexes". Organometallics. 15 (22): 4763. doi:10.1021/om960505r.
- Berkessel, Albrecht; Brandenburg, Marc; Leitterstorf, Eva; Frey, Julia; Lex, Johann; Schäfer, Mathias (2007). "A Practical and Versatile Access to Dihydrosalen (Salalen) Ligands: Highly Enantioselective Titanium. In Situ Catalysts for Asymmetric Epoxidation with Aqueous Hydrogen Peroxide". Adv. Synth. Catal. 349 (14–15): 2385. doi:10.1002/adsc.200700221.
- Pang, Xuan; Duan, Ranlong; Li, Xiang; Sun, Zhiqiang; Zhang, Han; Wang, Xianhong; Chen, Xuesi (2014). "Synthesis and characterization of half-salen complexes and their application in the polymerization of lactide and ε-caprolactone". Polymer Chemistry. 5 (23): 6857–6864. doi:10.1039/C4PY00734D.
- Wu, Xianghong, Gorden, A. V. E. (2009). "2-Quinoxalinol Salen Copper Complexes for Oxidation of Aryl Methylenes". Eur. J. Org. Chem. 2009 (4): 503–509. doi:10.1002/ejoc.200800928.CS1 maint: multiple names: authors list (link)
