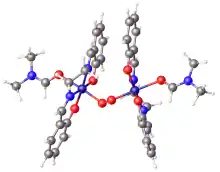
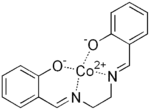
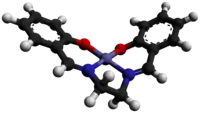
Salcomine
Salcomine is a coordination complex derived from the salen ligand and cobalt. The complex, which is planar, and a variety of its derivatives are carriers for O2 as well as oxidation catalysts.[2]
 | |
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.541 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H14CoN2O2 | |
| Molar mass | 325.233 g·mol−1 |
| Hazards | |
| Safety data sheet | Oxford MSDS |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S26 S36 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and structure
Salcomine is commercially available. It may be synthesized from cobalt(II) acetate and salenH2.[3]
Salcomine crystallizes as a dimer. In this form, the cobalt centers achieve five-coordination via a bridging phenolate ligands.[4] A monomeric form crystallizes with chloroform in the lattice. It features planar Co centers.[5] Salcomine is both a Lewis acid and a reductant. Several solvated derivatives bind O2 to give derivatives of the type (μ-O2)[Co(salen)py]2 and [Co(salen)py(O2)].[2]
Applications
The 1938 report that this compound reversibly bound O2[7] led to intensive research on this and related complexes for the storage or transport of oxygen. Solvated derivatives of salcomine, e.g. the chloroformate or the DMF adduct, bind 0.5 equivalent of O2:
- 2 Co(salen) + O2 → [Co(salen)]2O2
Salcomine catalyzes the oxidation of 2,6-disubstituted phenols by dioxygen.[8]
References
- N,N′-Bis(salicylidene)ethylenediaminocobalt(II) at Sigma-Aldrich
- Shoichiro Yamada “Advancement in stereochemical aspects of Schiff base metal complexes” Coordination Chemistry Reviews 1999, volume 190–192, 537–555.
- Appleton, T. G. (1977). "Oxygen Uptake by a Cobalt(II) Complex". J. Chem. Educ. 54 (7): 443. doi:10.1021/ed054p443.
- Bruckner, S.; Calligaris, M.; Nardin, G.; Randaccio, L. (1969). "The Crystal Structure of the Form of N,N-Ethylenebis(salicylaldehydeiminato)cobalt(II) Inactive Towards Oxygenation". Acta Crystallographica Section B. 25 (8): 1671-1674. doi:10.1107/S0567740869004523.CS1 maint: uses authors parameter (link)
- Schaefer, W. P.; Marsh, R. E. (1969). "Oxygen-Carrying Cobalt Compounds. I. Bis(salicylaldehyde)ethylenediiminecobalt(II) Monochloroformate". Acta Crystallographica Section B. 25 (9): 1675-1682. doi:10.1107/S0567740869004547.CS1 maint: uses authors parameter (link)
- M.Calligaris, G.Nardin, L.Randaccio, A.Ripamonti (1970). "Structural Aspects of the Synthetic Oxygen-Carrier NN′-Ethylenebis(Salicylideneiminato)cobalt(II): Structure of the Addition Compound with Oxygen Containing Dimethylformamide". J. Chem. Soc. A: 1069. doi:10.1039/j19700001069.CS1 maint: uses authors parameter (link)
- Tokuichi Tsumaki (1938). "Nebenvalenzringverbindungen. IV. Über einige innerkomplexe Kobaltsalze der Oxyaldimine". Bulletin of the Chemical Society of Japan. 13 (2): 252–260. doi:10.1246/bcsj.13.252.
- C. R. H. I. De Jonge, H. J. Hageman, G. Hoentjen, and W. J. Mijs (1988). "Oxidation with Bis(Salicylidene)ethylenediiminocobalt(II) (Salcomine): 2,6-Di-''tert''-butyl-''p''-benzoquinone". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 6, p. 412