Potassium pyrosulfate
Potassium pyrosulfate, or potassium disulfate, is an inorganic compound with the chemical formula K2S2O7.
 | |
| Names | |
|---|---|
| IUPAC name
dipotassium (sulfonatooxy)sulfonate | |
| Other names
Potassium pyrosulphate; potassium disulfate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.288 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| K2O7S2 | |
| Molar mass | 254.31 g·mol−1 |
| Density | 2.28 g/cm3 |
| Melting point | 325 °C (617 °F; 598 K) |
| soluble | |
| Hazards | |
| GHS pictograms | 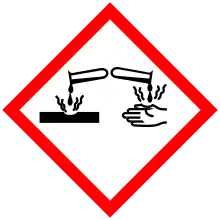  |
| GHS Signal word | Danger |
| H314, H318, H331 | |
| P260, P261, P264, P271, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P311, P321, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Potassium pyrosulfate is obtained by the thermal decomposition of other salts, most directly from potassium bisulfate:[1]
- 2 KHSO4 → K2S2O7 + H2O
Temperatures above 600°C further decompose potassium pyrosulfate to potassium sulfate and sulfur trioxide however:[2]
- K2S2O7 → K2SO4 + SO3
Other salts, such as potassium trisulfate,[3] can also decompose into potassium pyrosulfate.
Chemical structure
Potassium pyrosulfate contains the pyrosulfate anion which has a dichromate like structure. The geometry can be visualized as a tetrahedron with two corners sharing the SO4 anion's configuration and a centrally bridged oxygen atom.[4] A semi-structural formula for the pyrosulfate anion is O3SOSO32−. The oxidation state of sulfur in this compound is +6.
Uses
Potassium pyrosulfate is used in analytical chemistry; samples are fused with potassium pyrosulfate, (or a mixture of potassium pyrosulfate and potassium fluoride) to ensure complete dissolution prior to a quantitative analysis.[5][6]
The compound is also present in a catalyst in conjunction with vanadium(V) oxide in the industrial production of sulfur trioxide.[7]
See also
References
- Washington Wiley, Harvey (1895). Principles and Practice of Agricultural Analysis: Fertilizers. Easton, PA.: Chemical Publishing Co. p. 218. Retrieved 31 December 2015.
Potassium disulfate.
- Iredelle Dillard Hinds, John (1908). Inorganic Chemistry: With the Elements of Physical and Theoretical Chemistry. New York: John Wiley & Sons. p. 547. Retrieved 31 December 2015.
Potassium disulfate.
- Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry Vol. 2, 2nd Ed. New York: Academic Press. p. 1716. ISBN 9780323161299.
- Ståhl, K.; Balic-Zunic, T.; da Silva, F.; Eriksen, K. M.; Berg, R. W.; Fehrmann, R. (2005). "The crystal structure determination and refinements of K2S2O7, KNaS2O7 and Na2S2O7 from X-ray powder and single crystal diffraction data". Journal of Solid State Chemistry. 178 (5): 1697–1704. Bibcode:2005JSSCh.178.1697S. doi:10.1016/j.jssc.2005.03.022.
- Trostbl, L. J.; Wynne, D. J. (1940). "Determination of quartz (free silica) in refractory clays". Journal of the American Ceramic Society. 23 (1): 18–22. doi:10.1111/j.1151-2916.1940.tb14187.x.
- Sill, C. W. (1980). "Determination of gross alpha, plutonium, neptunium, and/or uranium by gross alpha counting on barium sulphate". Analytical Chemistry. 52 (9): 1452–1459. doi:10.1021/ac50059a018.
- Burkhardt, Donald (1965). "Sulfur trioxide production, US3362786A". Google Patents. Retrieved 31 December 2015.