Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme
The Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S) are two international instruments between countries and pharmaceutical inspection authorities. The PIC/S is meant as an instrument to improve co-operation in the field of Good Manufacturing Practices between regulatory authorities and the pharmaceutical industry.
 Logo of the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme | |
| Abbreviation | PIC/S |
|---|---|
| Formation | 26 May 1971 |
| Type | Intergovernmental organization |
| Purpose | Pharmaceutical |
| Headquarters | Geneva, Switzerland |
| Coordinates | 46.2050823°N 6.158363°E |
Area served | International |
Membership | 52 Active state members (+2 future Active state members) |
Secretariat |
|
| Website | www |
The PIC (Pharmaceutical Inspection Convention) was founded in October 1970 by the European Free Trade Association (EFTA), under the title of the Convention for the Mutual Recognition of Inspections in Respect of the Manufacture of Pharmaceutical Products. The initial members comprised the 10 member countries of EFTA at that time. In the early 1990s it was realized that because of an incompatibility between the Convention and European law, it was not possible for new countries to be admitted as members of PIC. European law did not permit individual EU countries that were members of PIC to sign agreements with other countries seeking to join PIC. As a consequence the Pharmaceutical Inspection Co-operation Scheme was formed on 2 November 1995. The Pharmaceutical Inspection Co-operation Scheme is an informal agreement between health authorities instead of a formal treaty between countries. PIC and the PIC Scheme, which operate together in parallel, are jointly referred to as PIC/S. PIC/S became operational in November 1995.
Members
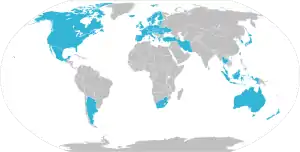
The following are the state members of PIC/S as of May 2020:[1]
| Country | Participating entity | Accession to PIC Scheme |
|---|---|---|
| Instituto Nacional de Medicamentos (INAME) | 2008 | |
| Therapeutic Goods Administration (TGA) | 1995 | |
| Österreichische Agentur für Gesundheit und Ernährungssicherheit (AGES) | 1999 | |
| Federal Agency for Medicines and Health Products (FAGG) | 1997 | |
| Health Canada's Regulatory Operations and Enforcement Branch (ROEB) | 1999 | |
| Food and Drug Administration (TFDA) | 2013 | |
| Agencija za lijekove i medicinske proizvode (HALMED) | 2016 | |
| Pharmaceutical Services (CyPHS) | 2008 | |
| State Institute for Drug Control (SÚKL) Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM) | 1997 2005 | |
| Danish Medicines Agency (DKMA) | 1995 | |
| State Agency of Medicines (SAM) | 2007 | |
| Finnish Medicines Agency (FIMEA) | 1996 | |
| Agence nationale de sécurité du médicament et des produits de santé (ANSM) Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES) | 1997 2009 | |
| Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) | 2000 | |
| Greek National Organisation for Medicines (EOF) | 2002 | |
| Pharmacy and Poisons Board of Hong Kong (PPBHK) | 2016 | |
| National Institute of Pharmacy and Nutrition (NIPN) | 1995 | |
| Icelandic Medicines Agency (IMA) | 1995 | |
| National Agency for Drug and Food Control (NADFC) | 2012 | |
| Iran Food and Drug Administration (IFDA) | 2018 | |
| Health Products Regulatory Authority (HPRA) | 1996 | |
| Institute for Standardization and Control of Pharmaceuticals (ISCP) | 2009 | |
| Italian Medicines Agency (AIFA) Direzione generale della sanità animale e dei farmaci veterinari (DGSAF) | 2000 2020 | |
| Pharmaceuticals and Medical Devices Agency (PMDA) | 2014 | |
| Zāļu valsts aģentūra (ZVA) | 2004 | |
| Amt für Gesundheit (AG) | 1995 | |
| State Medicines Control Agency (SMCA) | 2009 | |
| National Pharmaceutical Regulatory Agency (NPRA) | 2002 | |
| Malta Medicines Authority (MMA) | 2008 | |
| Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS) | 2018 | |
| Health and Youth Care Inspectorate | 1995 | |
| Medicines and Medical Devices Safety Authority (Medsafe) | 2013 | |
| Norwegian Medicines Agency (NOMA) | 1995 | |
| Chief Pharmaceutical Inspectorate (CPI) | 2006 | |
| Autoridade Nacional do Medicamento e Produtos de Saúde IP (INFARMED IP ) | 1999 | |
| National Agency for Medicines and Medical Devices (NAMMD) | 1995 | |
| Health Sciences Authority (HSA) | 2000 | |
| State Institute for Drug Control (SIDC) | 1997 | |
| Javna agencija Republike Slovenije za zdravila in medicinske pripomočke (JAZMP) | 2012 | |
| South African Health Products Regulatory Authority (SAHPRA) | 2007 | |
| Ministry of Food and Drug Safety (MFDS) | 2014 | |
| Spanish Agency of Medicines and Medical Devices (AEMPS) | 1998 | |
| Swedish Medical Products Agency (MPA) | 1996 | |
| Swiss Agency for Therapeutic Products (Swissmedic) | 1996 | |
| Food and Drug Administration (Thai FDA) | 2016 | |
| Turkish Medicines and Medical Devices Agency (TMMDA) | 2018 | |
| State Service for Medications and Drugs Control (SMDC) | 2011 | |
| Medicines and Healthcare products Regulatory Agency (MHRA) Veterinary Medicines Directorate (VMD) | 1999 2014 | |
| U.S. Food and Drug Administration (USFDA) | 2011 |
Future members
| Country | Participating entity | Accession to PIC Scheme |
|---|---|---|
| National Sanitary Surveillance Agency (Anvisa) | 2021 |
See also
- GxP
- Good Automated Manufacturing Practice (GAMP)
- Corrective and Preventative Action (CAPA)
- Validation (drug manufacture)
- European Medicines Agency (EMEA)
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- Pharmaceutical Research and Manufacturers of America (PhRMA)
- SAFE-BioPharma Association (SAFE)
References
- "Members". www.picscheme.org. Retrieved 17 May 2020.