Macimorelin
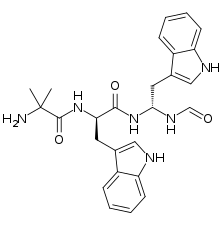
Macimorelin (INN), or Macrilen (trade name) is a drug being developed by Æterna Zentaris for use in the diagnosis of adult growth hormone deficiency. Macimorelin acetate, the salt formulation, is a synthetic growth hormone secretagogue receptor agonist.[2] Macimorelin acetate is described chemically as D-Tryptophanamide, 2-methylalanyl-N-[(1R)-1-(formylamino)-2-(1H-indol-3-yl)ethyl]-acetate.
 | |
| Clinical data | |
|---|---|
| Trade names | Macrilen |
| Other names | Aib-Trp-gTrp-CHO; AEZS-130; JMV 1843; Macimorelin acetate |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Elimination half-life | 4.1 hours |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H30N6O3 |
| Molar mass | 474.565 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
As of January 2014, it was in Phase III clinical trials.[3] The phase III trial for growth hormone deficiency is expected to be complete in December 2016.[4]
As of December 2017, it became FDA-approved as a method to diagnose growth hormone deficiency.[5] Traditionally, growth hormone deficiency was diagnosed via means of insulin tolerance test (IST) or glucagon stimulation test (GST). These two means are done parenterally, whereas Macrilen boasts an oral formulation for ease of administration for patients and providers.
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[6]
Macimorelin is a growth hormone secretagogue receptor (ghrelin receptor) agonist causing release of growth hormone from the pituitary gland.[7][8][9]
See also
References
- "Macimorelin Aeterna Zentaris". European Medicines Agency (EMA). 13 November 2018. Retrieved 28 September 2020.
- "Macrilen Prescribing Information" (PDF). Retrieved 2018-07-25.
- "Aeterna Zentaris NDA for Macimorelin Acetate in AGHD Accepted for Filing by the FDA". Wall Street Journal. January 6, 2014.
- Clinical trial number NCT02558829 for "Validation of Macimorelin as a Test for Adult Growth Hormone Deficiency" at ClinicalTrials.gov
- Center for Drug Evaluation and Research. "Drug Approvals and Databases - Drug Trials Snapshots: Marcrilen". www.fda.gov. Retrieved 2018-07-25.
- New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Retrieved 16 September 2020.
- "Macimorelin". NCI Drug Dictionary. National Cancer Institute.
- Koch L (June 2013). "Growth hormone in health and disease: Novel ghrelin mimetic is safe and effective as a GH stimulation test". Nature Reviews. Endocrinology. 9 (6): 315. doi:10.1038/nrendo.2013.89. PMID 23591367. S2CID 10475359.
- Garcia JM, Swerdloff R, Wang C, Kyle M, Kipnes M, Biller BM, et al. (June 2013). "Macimorelin (AEZS-130)-stimulated growth hormone (GH) test: validation of a novel oral stimulation test for the diagnosis of adult GH deficiency". The Journal of Clinical Endocrinology and Metabolism. 98 (6): 2422–9. doi:10.1210/jc.2013-1157. PMC 4207947. PMID 23559086.
External links
- "Macimorelin". Drug Information Portal. U.S. National Library of Medicine.
- "Macimorelin acetate". Drug Information Portal. U.S. National Library of Medicine.