Ice VI
Ice VI is a form of ice that exists at high pressure at the order of about 1 GPa (= 10 000 bar) and temperatures ranging from 130 up to 355 Kelvin (−143°C up to 82°C); see also the phase diagram of water. Its discovery and the discovery of other high pressure forms of water was published by P.W. Bridgman in January 1912.[1][2]
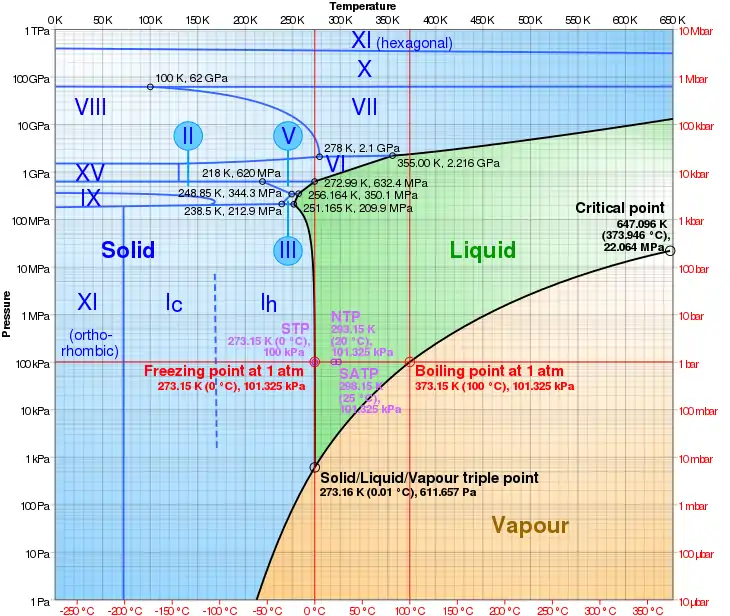
Phase diagram of water
Properties
Ice VI has a density of 1.31 g/cm³ and a tetragonal crystal system with the space group P42/nmc; its unit cell contains 10 water molecules and has the dimensions a=6.27 Å and c=5.79 Å.[4] The triple point of ice VI with ice VII and liquid water is at about 82°C and 2.22 GPa and its triple point with ice V and liquid water is at 0.16°C and 0.6324 GPa = 6324 bar.[5]
See also
External links
- Physik des Eises (PDF in German, iktp.tu-dresden.de)
- Ice phases (www.idc-online.com)
References
- Water, in the Liquid and Five Solid Forms, under Pressure P.W. Bridgman (1912), www.jstor.org, retrieved 3 October 2019
- Detailed crystallographic analysis of the ice VI to ice XV hydrogen ordering phase transition C.G. Salzmann (2016), aip.scitation.org, full text on arxiv.org
- https://youtube.com/KbXvJhjO_tQ?t=224
- Reports: Structure of Ice VI science.sciencemag.org, B. Kamb, 8 October 1965.
- Water Phase Diagram www1.lsbu.ac.uk, version of 9 September 2019, retrieved 3 October 2019
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.