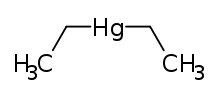
Diethylmercury
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous.[1] This chemical can cross the blood–brain barrier, causing permanent brain damage. It is, however, considerably less toxic than dimethylmercury.
 | |
| Names | |
|---|---|
| IUPAC name
diethylmercury | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.001 |
| EC Number |
|
| MeSH | C007378 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H10Hg | |
| Molar mass | 258.71 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet |
| Density | 2.446 g/ml |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 156 to 157 °C (313 to 315 °F; 429 to 430 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H300, H310, H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P304+340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+233, P405, P501 | |
| Flash point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Diethylmercury can be obtained from the reaction between ethylmagnesium bromide and mercury(II) chloride.[2]
- 2 C2H5MgBr + HgCl2 → Hg(C2H5)2 + MgBr2 + MgCl2
Other methods are also known.[3]
See also
- Dimethylmercury, a related compound
- Ethylmercury
- Mercury poisoning
References
- http://www.chemicalbook.com/ChemicalProductProperty_EN.aspx?CBNumber=CB7165894
- Brauer, Georg. Handbuch der präparativen anorganischen Chemie Bd. 2. Baudler, Marianne (3rd ed.). Stuttgart. p. 1063. ISBN 978-3-432-87813-3. OCLC 310719490.
- Kolbe, Hermann (1860). Ausführliches Lehrbuch der organischen Chemie, Volume 2. p. 964.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.