Deinacrida connectens
Deinacrida connectens, often referred to as the alpine scree wētā, is one of New Zealand’s largest alpine invertebrates and is a member of the Anostostomatidae family. Deinacrida connectens is a flightless nocturnal insect that lives under rocks at high elevation. Mountain populations vary in colour. This species is the most widespread of the eleven species of giant wētā (Deinacrida).
| Alpine scree wētā | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Orthoptera |
| Suborder: | Ensifera |
| Family: | Anostostomatidae |
| Genus: | Deinacrida |
| Species: | D. connectens |
| Binomial name | |
| Deinacrida connectens (Ander, 1939) | |
| Synonyms | |
| |
Taxonomy
Deinacrida connectens was originally described in 1939 by Swedish entomologist Kjell Ernst Viktor Ander as Deinacridopsis connectens, which was the only species of Deinacridopsis.[1] However, Deinacridopsis was later recognized as a synonym of Deinacrida by New Zealand entomologist Graeme William Ramsay in 1961 and the species was moved to the Deinacrida genus.[2] In the same paper, Ramsay also recognized Deinacrida sonitospina as a synonym of D. connectens, which was previously described by New Zealand entomologist John Tenison Salmon in 1950 from specimens found at Mount Peel and Mount Arthur.[2][3]
Habitat/distribution
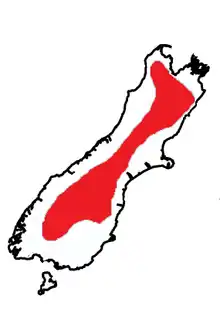
Deinacrida connectens is restricted to the South Island of New Zealand, where its distribution extends from the Arthur Range in North West Nelson to the Takitimu Range in Southland.[4] The range of D. connectens is known to overlap with the range of Deinacrida pluvialis in the western Otago mountains.[5] This relatively widespread species distribution is unusual in the Deinacrida genus, where species usually have a restricted distribution.[4] D. connectens generally inhabits scree slopes in alpine zones at elevations between 1200m and 3600m above sea level, but juveniles have also been found at 990m above sea level.[3][4] It is not known what factor restricts them to this zone.[6] The lower limit for their elevation range isolates populations on each mountain range.[6]D. connectens has been alleged to be the most abundant species of Deinacrida.[7]
Conservation
Under the 2014 New Zealand Threat Classification System, Deinacrida connectens is listed as a “Not Threatened” species and is the only species of Deinacrida not totally protected by legislation.[8][9]
Due to being restricted to high elevation, it is thought that introduced mammalian predators are not a threat to D. connectens populations, since these predators are uncommon in these regions.[6] This is in contrast to other Deinacrida species, which are generally at lower elevation and are more frequently preyed upon by introduced predators, causes a decline in giant wētā species abundance.[10]
Diet
Deinacrida connectens is known to be omnivorous, but in the wild is generally observed feeding on plants.[11] D. connectens has been observed in the wild browsing lichens, herbs and shrubs such as Aciphylla and Gaultheria depressa.[11][12] In captivity D. connectens ate a range of “vegetables" (lettuce, carrot, clover, and dandelion leaves), fruit (apples, apricots), and raw beef, cheese and insects (cicadas, tenebrionid beetles).[11] Feeding generally occurs early at night, after D. connectens has emerged from their daytime cover.[11]
Ecology
Deinacrida connectens is New Zealand’s largest nocturnal alpine insect, but occurs at lower abundance than smaller grasshopper and cockroach species in the same environment.[13] The scree wētā is known to be capable of dispersing some fleshy fruit seeds by endozoochory.[12] In an experiment, D. connectens' ability to disperse seeds of Gaultheria depressa by feeding was found to be dependent on the size of the wētā.[12] At smaller sizes, fewer seeds were eaten and the wētā could be considered seed predators, (almost no seeds made it intact through the guts of individuals measuring 2 cm or less).[12] With larger sized wētā however, thousands of seeds were consumed, some of which were presumably capable of being dispersed large distances, suggesting D. connectens can act as a seed disperser.[12] One captive individual D. connectens was recorded successfully passing 686 intact seeds.[12]
Morphology
Males of Deinacrida connectens, like other Deinacrida, are smaller than the females.[11][14] Adult males are about 3.5 cm in length whereas adult females are about 4.5 cm in length.[3] However, one source has described females measuring 7.2 cm long.[15] Body weight of adults has been recorded to reach almost 10g.[12]
Like other Anostostomatidae, D. connectens can produce sound by using their hind legs to rub their abdominal tergites.[16] The hind legs rub against tiny "peg" like structures, referred to as stridulatory pegs.[16]
Throughout the distribution of D. connectens, body colour shows extreme variation.[4][9] In some populations, individuals may have mostly black bodies (for example, those in a population located at Spence Peak in Southland) whereas others may have a mix of red, grey and olive colours.[4][9]


Breeding
The long legs of males compared to females suggest that like other members of this genus the scree wētā has a scramble competition mating system, in which adult females signal and males search for mates.[17] However, males of Deinacrida connectens appear to invest little energy into reproductive behaviours, and provide small spermatophores.[11] During mating in experimental conditions, males remain beneath the female, with the pair facing the same direction and with their bodies creating an angle of 30°.[11] Copulation has been observed to last around 35 minutes.[11] Mating would be concluded once the male walked away.[11]
There is a single observation in experimental conditions of a male scree wētā attempting to separate a mating pair.[11]
Behaviour
During the day, Deinacrida connectens remains under rocks and in crevices of scree slopes.[11] They may nestle with other conspecifics during this time.[11] During the night, D. connectens comes out of cover to feed on vegetation.[11]
When disturbed, D connectens will either remain motionless or attempt to run away and if they need to defend themselves, they will raise their legs in a threatening posture and produce soft sounds.[3][11] These sounds have been described as “soft and sibilant, rather like that produced by brushing together the heels of one's palms”.[11] D. connectens has been described as an aggressive species, and will bite if provoked (although they do not appear to be strong enough to break skin).[3]
After feeding, D. connectens will engage in “perching” behaviour, where it stands at the peak of a rock for extended periods of time during the night.[11]
In experimental conditions, individuals appeared to maintain an “individual distance” from one another while out at night.[11] This boundary was maintained by producing sound and using their hind legs to push and kick away other individuals that got too close.[11] However, this boundary does not seem to be maintained during the day, when individuals may huddle together under rocks and in crevices.[11]
In laboratory conditions (at temperatures higher than they normally experience), large D. connectens have been known to travel nearly 6 metres per minute.[12]
Phylogeography
Genetic diversity within Deinacrida connectens is quite high and partitioned between populations.[18][19]
In a phylogeography study of D. connectens, research found seven genetic lineages from mtDNA haplotypes, where each occupied a discrete geographic region.[7] The average genetic difference between haplotypes was 4.8%, which is quite high for an insect.[7] This phylogeographical structure combined with lineage age estimations suggests D. connectens radiated during the Pliocene mountain building that created the Southern Alps 5 million years ago.[7][19][20] It was also suggested that subsequent glaciation may have helped foster isolation between population of D. connectens.[7][20]
Physiology
Deinacrida connectens are adapted to be moderately freeze tolerant and are adapted to high elevation zones.[21][22]
Cytogenetics
Sex determination of most wētā species is by the number of large metacentric X-chromosomes; females have two X-chromosomes (XX) and males have one (X0).[23] The scree wētā is diploid with an even number of chromosomes in females, and an odd number in males, but populations within this species have different numbers of chromosomes.[4] There are seven known karyotypes within D. connectens.[4] These karyotypes vary in number of chromosomes from 2n = 17(X0) to 2n = 22(XX).[4] Known locations of these karyotype races are listed below.[4]
References
- Ander, K. 1939: Vergleichend-anatomische und phylogenetische Studien tiber die Ensifera (Saltatoria). Opuscula Entomologica, Supplement 2.
- Ramsay, G.W. 1961: The synonymy and systematics of a genus and two species of New Zealand weta (Orthoptera: Stenopelmatidae: Henicinae). Proceedings of the Royal Entomological Society of London (B), 30: 85–89. doi:10.1111/j.1365-3113.1961.tb00169.x
- Salmon, J.T. 1950: Revision of the New Zealand wetas - Anostostominae (Orthoptera: Stenopelmatidae). Dominion Museum records in entomology, 1: 121–177. , Accessed from: Bibliography of New Zealand Terrestrial Invertebrates, 05/06/2019
- Morgan-Richards, M. & Gibb G.W. 1996: Colour, Allozyme and Karyotype Variation Show Little Concordance in the New Zealand Giant Scree Weta Deinacrida Connectens (Orthoptera: Stenopelmatidae). Hereditas, 125(2-3): 265-276. doi:10.1111/j.1601-5223.1996.00265.x
- G.W. Gibbs. 2001: Habitats and Biogeography of New Zealand’s Deinacridine and Tusked Weta Species. In L.H. Field (Eds.), Biology of Wetas, King Crickets and their Allies (pp. 35-55). New York, USA: CABI Publishing. ISBN 9780851994086
- Trewick, S.A., Wallis, G.P. & Morgan-Richards, M. 2001: Phylogeographical pattern correlates with Pliocene mountain building in the alpine scree weta (Orthoptera, Anostostomatidae). Molecular Ecology, 9(6): 657-666. doi:10.1046/j.1365-294x.2000.00905.x
- Trewick, Steven A. (2001-01-01). "Scree weta phylogeography: Surviving glaciation and implications for Pleistocene biogeography in New Zealand". New Zealand Journal of Zoology. 28 (3): 291–298. doi:10.1080/03014223.2001.9518271. ISSN 0301-4223.
- Trewick, S.A., Johns, P., Hitchmough, R., Rolfe, J. & Stringer, I. 2014:Conservation status of New Zealand Orthoptera. New Zealand Threat Classification Series 16. ISSN 2324-1713
- "Alpine Scree Weta". WetaGeta.
- Gibbs, George W. (1998-12-01). "Why are some weta (Orthoptera: Stenopelmatidae) vulnerable yet others are common?". Journal of Insect Conservation. 2 (3): 161–166. doi:10.1023/A:1009660200402. ISSN 1572-9753.
- Field, L.H. 1980:Observations on the biology of Deinacrida connectens (Orthoptera: Stenopelmatidae), an alpine weta. New Zealand Journal of Zoology, 7(2):211-220 doi:10.1080/03014223.1980.10423778
- Larsen, H. & Burns, K.C. 2012:Seed dispersal effectiveness increases with body size in New Zealand alpine scree weta (Deinacrida connectens). Austral Ecology, 37(7): 800-806. doi:10.1111/j.1442-9993.2011.02340.x
- White, E (1975). "A survey and assessment of grasshoppers as herbivores in the South Island tussock grasslands of New Zealand". New Zealand Journal of Agriculture Research. 18: 73–85. doi:10.1080/00288233.1975.10430390.
- Richards, Aola M. (1973). "A comparative study of the biology of the Giant wetas Deinacrida heteracantha and D. fallai (Orthoptera : Henicidae) from New Zealand". Journal of Zoology. 169 (2): 195–236. doi:10.1111/j.1469-7998.1973.tb04554.x.
- Patrick, B.H. (1991). "Insects of the Dansey Ecological District" (PDF). Department of Conservation. Retrieved 19 June 2019.
- Gibbs, George W.; Morgan-Richards, Mary (2001). "A phylogenetic analysis of New Zealand giant and tree weta (Orthoptera : Anostostomatidae : Deinacrida and Hemideina) using morphological and genetic characters". Invertebrate Systematics. 15 (1): 1–12. doi:10.1071/it99022. ISSN 1447-2600.
- Kelly, Clint D.; Bussière, Luc F.; Gwynne, Darryl T. (2008). "Sexual Selection for Male Mobility in a Giant Insect with Female‐Biased Size Dimorphism". The American Naturalist. 172 (3): 417–423. doi:10.1086/589894. hdl:1893/914. ISSN 0003-0147. PMID 18651830.
- Morgan-Richards, Mary; Wallis, Graham P.; Trewick, Steven A. (September 2011). "The Invertebrate Life of New Zealand: A Phylogeographic Approach". Insects. 2 (3): 297–325. doi:10.3390/insects2030297. PMC 4553545. PMID 26467729.
- Trewick, S. A.; Wallis, G. P.; Morgan‐Richards, M. (2000). "Phylogeographical pattern correlates with Pliocene mountain building in the alpine scree weta (Orthoptera, Anostostomatidae)". Molecular Ecology. 9 (6): 657–666. doi:10.1046/j.1365-294x.2000.00905.x. ISSN 1365-294X. PMID 10849282.
- Brockie, Bob (24 September 2007). "Radiation of wētā species". Te Ara-The Encyclopedia of New Zealand. Retrieved 19 June 2019.
- Sinclair, B.J (1999). "Insect cold tolerance: How many kinds of frozen?" (PDF). European Journal of Entomology. 96: 157–164.
- Wharton, David A. (2011-08-01). "Cold tolerance of New Zealand alpine insects". Journal of Insect Physiology. "Cold and Desiccation Tolerance" honoring Karl Erik Zachariassen. 57 (8): 1090–1095. doi:10.1016/j.jinsphys.2011.03.004. ISSN 0022-1910. PMID 21397607.
- Mckean, N. E.; Trewick, S. A.; Morgan-Richards, M. (2015). "Comparative cytogenetics of North Island tree wētā in sympatry". New Zealand Journal of Zoology. 42 (2): 73–84. doi:10.1080/03014223.2015.1032984. ISSN 0301-4223.
| Wikispecies has information related to Deinacrida connectens. |