Borabenzene
Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike, the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent [C5H5BH]−.
| Names | |
|---|---|
| Other names
Borinine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H5B | |
| Molar mass | 75.91 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Adducts
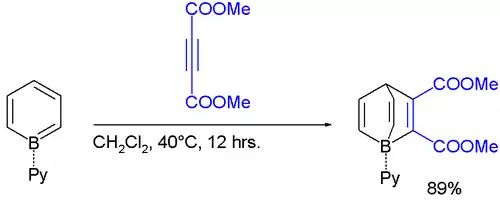
Adducts of borabenzene with Lewis bases are stable. Since borabenzene is unavailable, these adducts require indirect methods. 4-Silyl-1-methoxyboracyclohexadiene is used as a precursor to the borabenzene:
- C5H5N + MeOBC5H5SiMe3 → C5H5N-BC5H5 + MeOSiMe3
The pyridine adduct C5H5N-BC5H5 is structurally related to biphenyl.[1] It is a yellow solid. The pyridine ligand is tightly bound: no exchange is observed with deuterated pyridine at elevated temperatures.
The borabenzene-pyridine adduct behaves like a dienes, not an analogue of biphenyl.[2]
See also
- 6-membered aromatic rings with one carbon replaced by another group: borabenzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, stibabenzene, bismabenzene, pyrylium, thiopyrylium, selenopyrylium, telluropyrylium
- Borazine
References
- Boese, Roland; Finke, Norbert; Henkelmann, Jochem; Maier, Günther; Paetzold, Peter; Reisenauer, Hans Peter; Schmid, Günter (1985). "Synthese und Strukturuntersuchung von Pyridin-Borabenzol und Pyridin-2-Boranaphthalin". Chemische Berichte. 118 (4): 1644–1654. doi:10.1002/cber.19851180431.
- Wood, Thomas K.; Piers, Warren E.; Keay, Brian A.; Parvez, Masood (2006). "1-Borabarrelene Derivatives via Diels−Alder Additions to Borabenzenes". Organic Letters. 8 (13): 2875–2878. doi:10.1021/ol061201w. PMID 16774279.