Acylphosphatase
In enzymology, an acylphosphatase (EC 3.6.1.7) is an enzyme that catalyzes the following chemical reaction:[3]
| acylphosphatase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 3.6.1.7 | ||||||||
| CAS number | 9012-34-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Acylphosphatase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
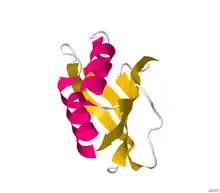
 Structure of acylphosphatase.[2] | |||||||||||
| Identifiers | |||||||||||
| Symbol | Acylphosphatase | ||||||||||
| Pfam | PF00708 | ||||||||||
| InterPro | IPR001792 | ||||||||||
| PROSITE | PDOC00136 | ||||||||||
| SCOP2 | 1aps / SCOPe / SUPFAM | ||||||||||
| |||||||||||
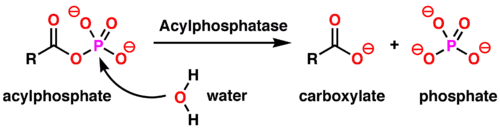
Thus, the two substrates of this enzyme are acylphosphate and H2O, whereas its two products are carboxylate and phosphate.
Function
This enzyme belongs to the family of hydrolases, specifically those acting on acid anhydrides in phosphorus-containing anhydrides. The systematic name of this enzyme class is acylphosphate phosphohydrolase. Other names in common use include acetylphosphatase, 1,3-diphosphoglycerate phosphatase, acetic phosphatase, Ho 1-3, and GP 1-3.
This enzyme participates in 3 metabolic pathways:
- glycolysis / gluconeogenesis
- pyruvate metabolism, and
- benzoate degradation via coa ligation.
Structural studies
Structures of this enzyme have been solved by both NMR and X-ray crystallography. See the links to PDB structures in the info boxes on the right for a current list of structures available in the PDB. The protein contains a beta sheet stacked on two alpha helices described by CATH as an Alpha-Beta Plait fold. The active site sits between sheet and helices and contains an arginine and an asparagine.[4] Most structures are monomeric [5]
Isozymes
Humans express the following two acylphosphatase isozymes:
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- "RCSB Protein Data Bank - Structure Summary for 2W4P - HUMAN COMMON-TYPE ACYLPHOSPHATASE VARIANT, A99G".
- Pastore A, Saudek V, Ramponi G, Williams RJ (March 1992). "Three-dimensional structure of acylphosphatase. Refinement and structure analysis". J. Mol. Biol. 224 (2): 427–40. doi:10.1016/0022-2836(92)91005-A. PMID 1313885.
- Stefani M, Taddei N, Ramponi G (February 1997). "Insights into acylphosphatase structure and catalytic mechanism". Cell. Mol. Life Sci. 53 (2): 141–51. doi:10.1007/PL00000585. PMID 9118002. S2CID 24072481.
- Gribenko AV, Patel MM, Liu J, McCallum SA, Wang C, Makhatadze GI (February 2009). "Rational stabilization of enzymes by computational redesign of surface charge-charge interactions". Proceedings of the National Academy of Sciences of the United States of America. 106 (8): 2601–6. Bibcode:2009PNAS..106.2601G. doi:10.1073/pnas.0808220106. PMC 2650310. PMID 19196981.
- "Enzyme 3.6.1.7". PDBe Enzyme Browser.