11-Aminoundecanoic acid
11-Aminoundecanoic acid is an organic compound with the formula H2N(CH2)10CO2H. This white solid is classified as an amine and a fatty acid. 11-Aminoundecanoic acid is a precursor to Nylon-11.[1]
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.017.652 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H23NO2 | |
| Molar mass | 201.31 |
| Appearance | white solid |
| Density | 1,1720 g·cm−3 |
| Melting point | 188–191 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
As practiced by Arkema, 11-aminoundecanoic acid is prepared industrially from undecylenic acid, which is derived from castor oil.[2] The synthesis proceeds in four separate reactions:
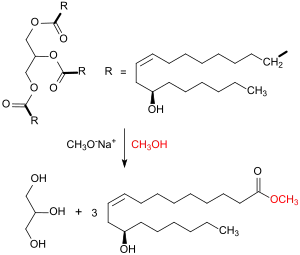
1. Transesterification of castor oil to methyl ricinoleat:
Crude castor oil consists of about 80% triglycerides, from the ricinoleic acid, itself representing about 90% of the oil.[3] It is quantitatively transesterified with methanol to methyl ricinoleat (the methyl ester of ricinoleic acid) in the presence of the basic sodium methoxide at 80 °C within 1 h reaction time in a stirred reactor. At the end of the reaction, the resulting glycerol separates and the liquid methyl ester is washed with water to remove residual glycerol.
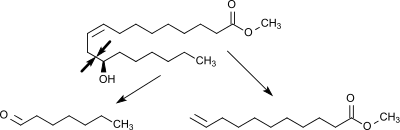
2. Pyrolysis of methylricinoleate to heptanal and methyl undecenoate:
Methylricinoleat is evaporated at 250 °C, mixed with hot steam (600 °C) in a 1:1 ratio and decomposed in a cracking furnace at 400 - 575 ° C at a retention time of about 10 seconds into its cleavage products heptanal and methyl undecenoate. The cleavage of the aliphatic chain occurs in this variant of the steam cracking selectively between the hydroxymethylene and the allyl-methylene group. Besides heptanal and methyl undecenoate, a mixture of methyl esters of saturated and unsaturated C18-carboxylic acids is obtained. This mixture is known under the trade name Esterol® and is used as a lubricant additive.
3. Hydrolysis of methyl undecenoate to 10-undecenoic acid
The hydrolysis of the methyl ester with sodium hydroxide proceeds at 25 °C within 30 min with quantitative yield. After acidification with hydrochloric acid, solid 10-undecenoic acid (undecylenic acid) is obtained.

4. Hydrobromination of 10-undecenoic acid to 11-bromoundecanoic acid
The undecenoic acid is dissolved in toluene and, in the presence of the radical initiator benzoyl peroxide (BPO), gaseous hydrogen bromide is added, in contrary to the Markovnikov rule ("anti-Markovnikov"). When cooled to 0 °C, the fast and highly exothermic reaction produces 11-bromoundecanoic acid in 95% yield - the Markownikov product 10-bromoundecanoic acid is produced in small quantities as a by-product. Toluene and unreacted hydrogen bromide are extracted under reduced pressure and resused.
![]()
5. Bromine exchange of 11-bromoundecanoic acid to 11-aminoundecanoic acid
11-Bromodecanoic acid is mixed at 30 °C with a large excess of 40% aqueous ammonia solution. When the reaction is complete, water is added and the mixture is heated to 100 °C to remove the excess ammonia.
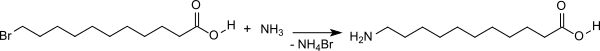
The acid can be recrystallized from water. For further purification, the hydrochloride of 11-aminoundecanoic acid, which is available by acidification with hydrochloric acid, can be recrystallized from a methanol/ethyl acetate mixture.[4]
Properties
11-aminoundecanoic acid is a white crystalline and odourless solid with low solubility in water.
Use
By acylation of 11-aminoundecanoic acid with chloroacetyl chloride, chloroacetylamino-11-undecanoic acid can be produced, which acts as a fungicide and insecticide.[5]
N-acyl derivatives of 11-aminoundecanoic acid in the form of oligomeric amides have remarkable properties as gelling agents for water and organic solvents.[6]
Monomer for polyamide 11
By far the most important application of 11-aminoundecanoic acid is its use as a monomer for polyamide 11 (also: nylon-11). Wallace Carothers, the inventor of polyamide (nylon 66), is said to have polymerized 11-aminoundecanoic acid as early as 1931.[7]
![]()
Although polyamide 11 is derived from a renewable raw material (i.e. biobased), it is not biodegradable. Nevertheless, it has the most advantageous ecological profile of comparable thermoplastics.[8] Due to its excellent toughness at low temperatures, polyamide 11 can be used at temperatures as low as -70 °C. Its relatively non-polar molecular structure due to the low frequency of amide bonds in the molecule results in low moisture absorption compared to polyamide 6 or polyamide 66. In addition, polyamide 11 has very good chemical stability, e.g. against hydrocarbons, low density, good thermal stability, weather resistance and is easy to process.
References
- Ben Herzog, Melvin I. Kohan, Steve A. Mestemacher, Rolando U. Pagilagan and Kate Redmond "Polyamides" in Ullmann's Encyclopedia of Industrial Chemistry 2013, Wiley-VCH, Weinheim. doi:10.1002/14356007.a21_179.pub3.
- A. Chauvel, G. Lefebvre, Petrochemical Processes: Technical and Economic Characteristics, Band 2, S. 277, Editions Technip, Paris, 1989, ISBN 2-7108-0563-4.
- Gupta, S. S.; Hilditch, T. P.; Riley, J. P. (1951). "The fatty acids and glycerides of castor oil". Journal of the Science of Food and Agriculture. 2 (6): 245–251. doi:10.1002/jsfa.2740020603.
- M.-H. Koh et al.: Divergent process for C10, C11, and C12 α-amino acid and α,ω-dicarboxylic acid monomers of polyamides from castor oil as a renewable resource. In: Bull. Korean Chem. Soc., 33 (6), 1873–1878 (2012), doi:10.5012/bkcs.2012.33.6.1873.
- US 4055663, Sol J. Barer et al., abgetreten an National Patent Development Corp., "Halogenated acylamino acids as fungicides", issued 1977-10-25
- A. D’Aleo et al., 11-Aminoundecanoic acid: a versatile unit for the generation of low molecular weight gelators for water and organic solvents, Chem. Commun., 2004, 190–191, doi:10.1039/B307846A.
- Renewable Polymers: Synthesis, Processing, and Technology, edited by V. Mittal, J. Wiley & Sons, Hoboken, NJ, ISBN 978-0-470-93877-5
- Jim Mason: Rilsan Polyamide-11 - A success story for sustainable resource based engineering thermoplastics, 9. April 2008.