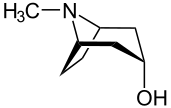
Tropine
Tropine is a derivative of tropane containing a hydroxyl group at the third carbon. It is also called 3-tropanol.[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(3-endo)-8-Methyl-8-azabicyclo[3.2.1]octan-3-ol | |||
| Other names
α-Tropine; Tropanol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.003.986 | ||
| MeSH | Tropine | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H15NO | |||
| Molar mass | 141.214 g·mol−1 | ||
| Appearance | Hygroscopic plates | ||
| Density | 1.016 g/cm3 at 100 °C | ||
| Melting point | 64 °C (147 °F; 337 K) | ||
| Boiling point | 233 °C (451 °F; 506 K) | ||
| Solubility | Very soluble in water, diethyl ether, ethanol[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tropine is a central building block of many chemicals active in the nervous system, including tropane alkaloids. Some of these compounds, such as long-acting muscarinic antagonists are used as medicines because of these effects.[2]
See also
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3–564, ISBN 0-8493-0594-2
- Ping, Yu; Li, Xiaodong; You, Wenjing; Li, Guoqiang; Yang, Mengquan; Wei, Wenping; Zhou, Zhihua; Xiao, Youli (10 June 2019). "Production of the Plant-Derived Tropine and Pseudotropine in Yeast". ACS Synthetic Biology. 8 (6): 1257–1262. doi:10.1021/acssynbio.9b00152.
- "Cocaine analog in two steps from native plant material".
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.