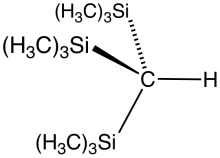
Tris(trimethylsilyl)methane
Tris(trimethylsilyl)methane is the organosilicon compound with the formula (tms)3CH (where tms = (CH3)3Si). It is a colorless liquid that is highly soluble in hydrocarbon solvents. Reaction of tris(trimethylsilyl)methane with methyl lithium gives tris(trimethylsilyl)methyllithium, called trisyllithium. Trisyllithium is useful in Petersen olefination reactions:[1]
- (tms)3CH + CH3Li → (tms)3CLi + CH4
- (tms)3CLi + R2CO → (tms)2C=CR2 + tmsOLi
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.154.179 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H28Si3 | |
| Molar mass | 232.589 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.827 g/cm3 |
| Boiling point | 219 °C (426 °F; 492 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trisyllithium is also an effective precursor to bulky ligands.
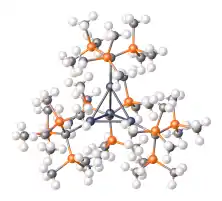
Structure of [InC(tms)3]4, an In(I) tetrahedrane (dark gray = In, orange = Si).[2]
See also
References
- Sakurai, Hideki (2001). "Tris(trimethylsilyl)methane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt417.
- Uhl, Werner; Graupner, Rene; Layh, Marcus; Schütz, Uwe (1995). "In4{C(SiMe3)3}4 mit In4-tetraeder und In4Se4{C(SiMe3)3}4 mit In4Se4-heterocubanstruktur". Journal of Organometallic Chemistry. 493 (1–2): C1–C5. doi:10.1016/0022-328X(95)05399-A.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.