Triptycene
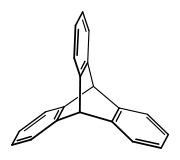
Triptycene is an aromatic hydrocarbon, the simplest iptycene molecule with the formula C2H2(C6H4)3. It is a white solid that is soluble in organic solvents. The compound has a paddle-wheel configuration with D3h symmetry. It is named after the medieval three-piece art panel, the triptych.[1] Several substituted triptycenes are known. Barrelenes are structurally related. Due to the rigid framework and three-dimensional geometry, derivatives of triptycene have been well researched.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
9,10-Dihydro-9,10-[1,2]benzenoanthracene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.837 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H14 | |
| Molar mass | 254.332 g·mol−1 |
| Melting point | 252 to 256 °C (486 to 493 °F; 525 to 529 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
The parent triptycene was first prepared in 1942 by a multistep method.[1] It can also be prepared in one step in 28% yield from the Diels–Alder reaction of anthracene and benzyne.[3] In this method, benzyne is generated by the reaction of magnesium and 2-bromofluorobenzene.
Derivatives and applications
The hydrocarbon framework is very rigid and triptycene derivatives such as triptycene quinones[4] are therefore incorporated in many organic compounds as a molecular scaffold for various applications, such as molecular motors[5] or ligands.
For example, a bis(diphenylphosphino) derivative was used as a phosphine ligand on nickel in a highly selective hydrocyanation reaction of butadiene:[6] The reactivity of this catalyst is attributed to the large bite angle of the bidentate ligand supported by the triptycene framework.
References
- Bartlett, Paul D.; Ryan, M. Josephine; Cohen, Saul G. (1942). "Triptycene1 (9,10-o-Benzenoanthracene)". J. Am. Chem. Soc. 64 (11): 2649–2653. doi:10.1021/ja01263a035.
- Zhao, Liwei; Li, Zhong; Wirth, Thomas (2010). "Triptycene Derivatives: Synthesis and Applications". Chemistry Letters. 39 (7): 658–667. doi:10.1246/cl.2010.658.
- Wittig, George (1959). "Triptycene". Org. Synth. 39: 75. doi:10.15227/orgsyn.039.0075.
- Spyroudis S, Xanthopoulou N (2003). "Triptycene quinones in synthesis: preparation of triptycene bis-cyclopentenedione" (PDF). Arkivoc. 2003 (vi): 95–105. doi:10.3998/ark.5550190.0004.612.
- Kelly TR, De Silva H, Silva RA (September 1999). "Unidirectional rotary motion in a molecular system". Nature. 401 (6749): 150–152. doi:10.1038/43639. PMID 10490021.
- Bini L, Müller C, Wilting J, von Chrzanowski L, Spek AL, Vogt D (October 2007). "Highly selective hydrocyanation of butadiene toward 3-pentenenitrile". J. Am. Chem. Soc. 129 (42): 12622–12623. doi:10.1021/ja074922e. hdl:1874/26892. PMID 17902667.