Trifluoroacetone
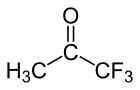
Trifluoroacetone (1,1,1-trifluoroacetone) is an organofluorine compound with the chemical formula CF3C(O)CH3.[1] The compound is a colorless liquid with chloroform-like odour.[2]
 | |
| Names | |
|---|---|
| IUPAC name
1,1,1-Trifluoropropan-2-one | |
| Other names
Trifluoracetone, TFA | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.370 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H3F3O | |
| Molar mass | 112.051 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.252 g/mL |
| Melting point | −78 °C (−108 °F; 195 K) |
| Boiling point | 21–24 °C (70–75 °F; 294–297 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
| H224, H315, H319, H335 | |
| P210, P261, P303, P351, P338 | |
| Flash point | −30 °C (−22 °F; 243 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation, reactions, uses
Trifluoroacetone is produced by decarboxylation of trifluoroacetoacetic acid:
- CF3C(O)CH2CO2H → CF3C(O)CH3 + CO2
The acetoacetic acid in turn is obtained via condensation of acetate and trifluoroacetate esters.[2]
Trifluoroacetone has been examined as oxidizing agent in Oppenauer oxidation, in which case hydroxyl groups of secondary alcohols can be oxidized in the presence of hydroxy groups of primary alcohols.[3]
Trifluoracetone is also used in a synthesis of 2-trifluoromethyl-7-azaindoles starting with 2,6-dihalopyridines. The derived chiral imine is used to prepare enantiopure α-trifluoromethyl alanines and diamines by a Strecker reaction followed by either nitrile hydrolysis or reduction.[4]
See also
References
- "1,1,1-Trifluoracetone 95%". dk.vwr.com. Retrieved 6 June 2017.
- Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349. ISBN 978-3527306732.CS1 maint: uses authors parameter (link)
- Mello, Rossella; Martínez-Ferrer, Jaime; Asensio, Gregorio; González-Núñez, María Elena (2007). "Oppenauer Oxidation of Secondary Alcohols with 1,1,1-Trifluoroacetone as Hydride Acceptor". J. Org. Chem. 24 (72): 9376–9378. doi:10.1021/jo7016422. PMID 17975928.
- "Concise synthesis of enantiopure alpha-trifluoromethyl alanines, diamines, and amino alcohols by the Strecker-type reaction". sigmaaldrich.com. Retrieved 6 June 2017.