Transalkylation
Transalkylation is a chemical reaction involving the transfer of an alkyl group from one organic compound to another. The reaction is used for the transfer of methyl and ethyl groups between benzene rings. This is of particular value in the petrochemical industry[1] to manufacture p-xylene, styrene,[2] and other aromatic compounds. Motivation for using transalkylation reactions is based on a difference in production and demand for benzene, toluene, and xylenes. Transalkylation can convert toluene, which is overproduced, into benzene and xylene, which are under-produced.[3] Zeolites are often used as catalysts in transalkylation reactions.[4]
Disproportionation
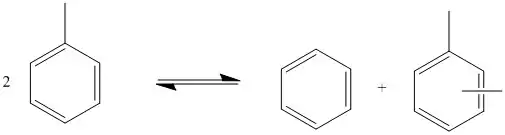
Transalkylation, as used by the petrochemical industry, is often used to convert toluene into benzene and xylenes. This is achieved through a disproportionation reaction of toluene in which one toluene molecule transfers its methyl group to another one. The reaction is not selective, and the xylene produced can be ortho, meta, or para. There is a higher demand for para xylene, so it is often separated, and the mixture is allowed to reequilibrate to give more para product.[3]
Diethylbenzenes
Diethylbenzenes arise as side-products of the alkylation of benzene with ethylene, which is conducted on a very large scale. Since there is only a limited market for diethylbenzene, much of it is recycled by transalkylation give ethylbenzene:[1]
- C6H4(C2H5)2 + C6H6 → 2 C6H5C2H5
M/R Ratio
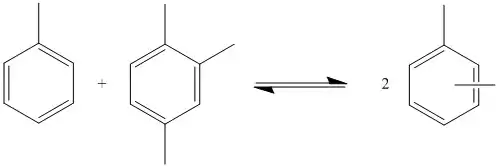
This type of reaction can also be performed with toluene and trimethylbenzene to produce xylene. The reaction occurs via equilibrium, so the product is not pure xylene. Many products are produced with varying numbers of methyl groups. The quantities in which each product is produced depends on the M/R ratio. This is the ratio of the number of methyl groups to the number of benzene rings in all of the substrates. For example, in the disproportionation of toluene, the M/R ratio is 1. Side reactions in which alkanes are produced reduce the number of methyl groups available which decreases the M/R ratio. This can be mitigated by adding compounds with higher numbers of methyl groups, such as trimethylbenzene. The ratio of products produced depends only on the M/R ratio so different starting materials can produce the same compounds via transalkylation.[3]
Zeolite catalysts
Transalkylation reactions of six to ten carbon methylated aromatics are often performed with the cofed of hydrogen gas, over a zeolite based solid catalyst. Industrial processes operate the transalkylation reactor at elevated temperature and pressure to achieve desired process economics. Zeolites are micro-crystalline solids composed of tetrahedral AlO
4 and SiO
4 building blocks. These crystals are porous in nature with characteristic micropore channels, cavities. Zeolite is known as one class of molecular sieve because of their channel openings are often between 0.4-1.5 nanometers, just enough for the molecules to pass through. Aromatics molecules enter and exit these channels at different rates, also called diffusion. In addition to their molecular sieving effect, zeolites have weakly bonded protons originated from its chemical composition. These are chemical active centers for acid-catalyzed transalkylation reaction.
Zeolites of varying sizes are used to perform transalkylation on different substrates. For example, zeolites with a pore size of 5.5Å are suitable for benzene, toluene, xylenes and trimethylbenzenes transalkylations.[3]
See also
References
- Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
- Styrene process with recycle from dehydrogenation zone Archived 2012-03-20 at the Wayback Machine
- Tsai, Tseng-Chang "Disproportionation and transalkylation of alkylbenzenes over zeolite catalysts". Elsevier Science, 1999
- US application 20100022814, Goncalvez De Almeida; Jose Luis & Berna Tejero et al., "Catalytic transalkylation of dialkyl benzenes", published 2010-01-28, assigned to Petroquimica Espanola, S.A. Petresa
- Oh, S.H.; Seong, K.H.; Kim, Y.S.; Choi, S.; Lim, B.S.; Lee, J.H.; Woltermann, J.; Chu, Y.F. (2002). "Reformate upgrading to produce enriched BTX using noble metal promoted zeolite catalyst". Studies in Surface Science and Catalysis. 142: 595–601. doi:10.1016/S0167-2991(02)80078-7. ISBN 9780444511744.