Thiothionyl fluoride
Thiothionyl fluoride is a chemical compound of fluorine and sulfur and an isomer of disulfur difluoride.
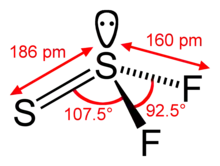 | |
| Names | |
|---|---|
| IUPAC name
difluoro(sulfanylidene)-λ4-sulfane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| F2S2 | |
| Molar mass | 102.12 g·mol−1 |
| Appearance | colorless gas |
| Melting point | −164.6 °C (−264.3 °F; 108.5 K) |
| Boiling point | −10.6 °C (12.9 °F; 262.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Thiothionyl fluoride can be obtained from the reaction between disulfur dichloride with potassium fluoride at about 150 °C or with mercury(II) fluoride at 20 °C.[1][2]
- S2Cl2 + 2 KF → SSF2 + 2 KCl
Another possible preparation is by the reaction of nitrogen trifluoride with sulfur.[1]
- NF3 + 3 S → SSF2 + NSF
It also forms from disulfur difluoride when in contact with alkali metal fluorides.[3]
Properties
Thiothionyl fluoride is a colorless gas.[2] At high temperatures and pressures, it decomposes into sulfur tetrafluoride and sulfur.[2]
- 2 SSF2 → SF4 + 3 S
With hydrogen fluoride, it forms sulfur tetrafluoride and hydrogen sulfide.[4]
- SSF2 + 2 HF → SF4, + H2S
References
- Brauer, Georg; Baudler, Marianne (1975). Handbuch der Präparativen Anorganischen Chemie. Band I. (3rd ed.). Stuttgart: Ferdinand Enke. p. 182. ISBN 3-432-02328-6.
- Holleman, A. F.; Wiberg, E.; Wiberg, N. (1995). Lehrbuch der Anorganischen Chemie (101st ed.). Berlin: Walter de Gruyter. p. 379. ISBN 3-11-012641-9.
- Steudel, Ralf (2008). Chemie der Nichtmetalle: Von Struktur und Bindung zur Anwendung. Walter de Gruyter. p. 475. ISBN 311021128-9.
- Kolditz, Lothar (1983). Anorganische Chemie. Berlin: Deutscher Verlag der Wissenchaften. p. 468.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.