Targeted intra-operative radiotherapy
Targeted intra-operative radiotherapy is a technique of giving radiotherapy to the tissues surrounding a cancer after its surgical removal, a form of intraoperative radiation therapy. The technique was designed in 1998 at the University College London.[1] In patients having lumpectomy for breast cancer, the TARGIT-A(lone) randomized controlled trial (recruitment from 2000–2012) tested whether TARGIT within a risk-adapted approach is non-inferior to conventional course of external beam postoperative radiotherapy given over several weeks.[2]
| Targeted intra-operative radiotherapy | |
|---|---|
| Other names | TARGIT |
| Specialty | oncology |
TARGIT is a method where the radiation is applied during an operation and targeted to the peri-tumoural tissues. TARGIT technique was designed at University College London[3] by Jayant S Vaidya (who coined the TARGIT acronym) and Michael Baum along with Jeffrey S Tobias in 1998. The term was first used when the technique was described,[4] and the protocol for a randomised trial was published by The Lancet.[5]
Medical uses
Breast cancer
The largest experience with IORT using the TARGIT technique and the best evidence for its potentials exists in breast cancer where a substantial number of patients have already been treated.[6]
Adoption
At the St Gallen Breast Cancer Conference (16–19 March 2011) the consensus amongst over 52 breast cancer expert panelists was that TARGIT alone could be used as the only radiation treatment in selected cases after breast conserving surgery (49% yes, 36% no), or as a tumour bed boost instead of external beam radiotherapy boost (62% yes, 23% no).[7]
On 25 July 2014 the UK National Institute for Health and Care Excellence (NICE) gave provisional recommendation for the use of TARGIT IORT with Intrabeam in the UK National Health Service.[8][9] In September 2014, NICE requested further information from the clinical trial investigators, citing several comments and concerns.[10] Concerns cited included the immaturity of the data with a median follow up of the entire population being only 2 years and 5 months as well as the noninferiority criterion used in the study.[11] This extra information was supplied by the authors, and has since been published as part of the comprehensive paper on TARGIT-A trial.[12] In 2017, NICE described it as an option for early breast cancer.[13]
The 2015 update of guidelines of the Association of Gynecological Oncology (AGO) (an autonomous community of the German Society of Gynecology and Obstetrics (DGGG) and the German Cancer Society) includes TARGIT IORT during lumpectomy as a recommended option for women with a T1, Grade 1 or 2, ER positive breast cancer.[14]
On 21 May 2015, the Australian Government Medical Services Advisory Committee (MSAC) announced that "After considering the available evidence in relation to safety, clinical effectiveness and cost-effectiveness, MSAC supported public funding of a new Medicare Benefits Schedule (MBS) item for treatment of pathologically documented invasive ductal breast cancer in eligible patients with TARGIT-IORT when used concurrently with breast-conserving surgery".[15] The Australian Government also approved budget item for the treatment of early stage breast cancer using targeted intraoperative radiotherapy[16] and patients can avail of this treatment from 1 September 2015 [17]
On 26 May 2015, in response to a query by the British Medical Journal, NICE clarified that while their appraisal is going on, TARGIT IORT with Intrabeam can continue to be offered to patients who need it [18]
There are over 250 centres worldwide using TARGIT IORT for treating breast cancer in USA (about 60 centres), Europe (60 centres in Germany), Australia, Middle East, Far East, South America. Over than 12,000 patients have been treated.[19] A recent study with several centres from the USA found excellent results with the use of TARGIT IORT.[20]
Evaluation of the long-term outcomes in patients who were treated with TARGIT-IORT for breast cancer confirmed that it is as effective as whole breast external beam radiotherapy in controlling cancer, and also reduces deaths from other causes [21][22] as shown in a large international randomised clinical trial published in the British Medical Journal.[23]
Rationale
When breast cancer is surgically excised, it can come back (local recurrence) in the remaining breast or on the chest wall in a small proportion of women. Adjuvant radiotherapy is necessary if breast cancer is treated by removing only the cancerous lump with a rim of surrounding normal tissue, as it reduces the chance of local recurrence significantly. When cancer does come back, it most commonly occurs in the tissues surrounding the original cancer (the tumour bed), even though there are multicentric cancers in remote areas of the breast. This suggests that it is most important to treat the tumour bed.[24] The rationale for TARGIT is to deliver a high dose of radiation precisely to the tumour bed. Conventional radiation techniques such as external beam radiotherapy (EBRT) following surgical removal of the tumor have been time tested and proven to be effective. EBRT is usually given as a course of whole breast radiotherapy and an additional tumour bed boost. However, it has a few drawbacks; for example, the tumour bed where the boost dose should be applied can be missed ("geographical miss") due to the difficulties in localization of the complex wound cavity even when modern radiotherapy planning is used. Additionally, the usual delay ("temporal miss") between the surgical removal of the tumour and EBRT may allow a repopulation of the tumour cells. These potentially harmful effects can be avoided by delivering the radiation more precisely to the targeted tissues leading to immediate sterilization of residual tumour cells. Furthermore, TARGIT inhibits the stimulating effects of wound fluid on cancer cells, suggesting for the first time, a beneficial effect of intraoperative radiotherapy (IORT) on tumour microenvironment.[25][26]
Technique
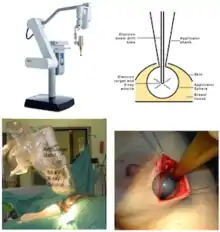
The machine used for TARGIT is Intrabeam (Carl Zeiss, Germany).[27] It is a miniature and mobile X-ray source which emits low energy X-ray radiation (max. 50 kV) in isotropic distribution. Due to the higher ionization density caused by soft X-ray radiation in the tissue, the relative biological effectiveness (RBE) of low-energy X-rays on tumour cells is higher when compared to high-energy X-rays or gamma rays which are delivered by linear accelerators.[28] The radiation which is produced by mobile radiation systems has a limited range. For this reason, conventional walls are regarded sufficient to stop the radiation scatter produced in the operating room and no extra measures for radiation protection are necessary. This makes IORT for breast cancer by the TARGIT technique available in most operating rooms. The surgical technique[29] is relatively simple but needs to be meticulously followed.[30]
Professional Society for Intraoperative Radiation Therapy
In 1998, the International Society of IORT (ISIORT) was formed to foster the scientific and clinical development of IORT. The ISIORT has more than 1000 members worldwide and meets every two years.[31]
See also
References
- "TARGIT: transforming the breast cancer treatment paradigm". University College London. 2014-12-14. Retrieved 26 July 2016.
- Vaidya, Jayant S; Wenz, Frederik; Bulsara, Max; et al. (February 2014). "Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial". The Lancet. 383 (9917): 603–613. doi:10.1016/S0140-6736(13)61950-9. PMID 24224997.
- Vaidya, Jayant S (2002). A Novel Approach to local treatment of breast cancer (PhD thesis). University of London.
- Vaidya JS, Baum M, Tobias JS, et al. (August 2001). "Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer". Ann. Oncol. 12 (8): 1075–80. doi:10.1023/A:1011609401132. PMID 11583188.
- Vaidya JS, Baum M, Tobias JS, Houghton J. "Targeted Intraoperative Radiotherapy (TARGIT)-trial protocol". The Lancet.
- Vaidya JS, Baum M, Tobias JS, et al. (December 2006). "Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost". Int. J. Radiat. Oncol. Biol. Phys. 66 (5): 1335–8. doi:10.1016/j.ijrobp.2006.07.1378. PMID 17084562.
- Gnant, Michael; Harbeck, Nadia; Thomssen, Christoph (2011). "St. Gallen 2011: Summary of the Consensus Discussion". Breast Care. 6 (2): 136–141. doi:10.1159/000328054. PMC 3100376. PMID 21633630.
- "NICE to recommend new breast cancer radiotherapy treatment alongside further research | Press and media | News". NICE. 2014-07-24. Retrieved 2016-05-18.
- Smyth, Chris (2014). "Single-dose radiotherapy eases breast cancer stress". The Times. Retrieved 26 July 2016.
- "Breast cancer (early) - intrabeam radiotherapy system [ID618]". NICE. Retrieved 26 July 2016.
- NICE concerns and requested actions for TARGIT Trialists
- Vaidya, Jayant S; Wenz, Frederik; Bulsara, Max; Tobias, Jeffrey S; Joseph, David J; Saunders, Christobel; Brew-Graves, Chris; Potyka, Ingrid; Morris, Stephen (2016). "An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial)". Health Technology Assessment. 20 (73): 1–188. doi:10.3310/hta20730. PMC 5056335. PMID 27689969.
- Wise, Jacqui (2017-02-10). "NICE recommends controlled intrabeam use for breast cancer after three year delay". BMJ. 356: j725. doi:10.1136/bmj.j725. ISSN 0959-8138. PMID 28188126.
- "Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer" (PDF). AGO. Retrieved 26 July 2016.
- "Application 1189 - Targeted intraoperative radiotherapy (T-IORT) for early breast cancer" (PDF). Medical Services Advisory Committee. Australian Government. Retrieved 26 July 2016.
- Health. "Budget Paper No. 2: Budget Measures - Part 2: Expense Measures - Health". Budget.gov.au. Retrieved 2016-05-18.
- "Revolution in Radiotherapy". The West Australian. 31 August 2015. Archived from the original on 5 March 2016. Retrieved 26 July 2016.
- Hawkes, N. (26 May 2015). "Start of cheaper technique for breast cancer is delayed in UK despite adoption elsewhere". BMJ. 350 (may26 14): h2874. doi:10.1136/bmj.h2874. PMID 26013648.
- "Worldwide adoption of TARGeted Intraoperative radioTherapy TARGIT IORT for breast cancer | Jayant S Vaidya". Jayantvaidya.org. 2015-10-22. Retrieved 2016-05-18.
- Valente, Stephanie A.; Tendulkar, Rahul D.; Cherian, Sheen; et al. (9 May 2016). "TARGIT-R (Retrospective): North American Experience with Intraoperative Radiation Using Low-Kilovoltage X-Rays for Breast Cancer". Annals of Surgical Oncology. 23 (9): 2809–2815. doi:10.1245/s10434-016-5240-1. PMID 27160524.
- Correspondent, Kat Lay, Health. "New treatment heralds breakthrough for breast cancer patients". ISSN 0140-0460. Retrieved 2020-09-09.
- "New breast cancer treatment requires just one shot of radiotherapy". The Independent. 2020-08-20. Retrieved 2020-09-17.
- Vaidya, Jayant S.; Bulsara, Max; Baum, Michael; Wenz, Frederik; Massarut, Samuele; Pigorsch, Steffi; Alvarado, Michael; Douek, Michael; Saunders, Christobel; Flyger, Henrik L.; Eiermann, Wolfgang (2020-08-19). "Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial". BMJ. 370. doi:10.1136/bmj.m2836. ISSN 1756-1833. PMID 32816842.
- Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I (September 1996). "Multicentricity of breast cancer: whole-organ analysis and clinical implications". Br. J. Cancer. 74 (5): 820–4. doi:10.1038/bjc.1996.442. PMC 2074702. PMID 8795588.
- Massarut S, Baldassare G, Belleti B, Reccanello S, D'Andrea S, Ezio C, Perin T, Roncadin M, Vaidya JS (2006). "Intraoperative radiotherapy impairs breast cancer cell motility induced by surgical wound fluid". J Clin Oncol. 24 (18S): 10611. doi:10.1200/jco.2006.24.18_suppl.10611.
- Belletti B, Vaidya JS, D'Andrea S, et al. (March 2008). "Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding". Clin. Cancer Res. 14 (5): 1325–32. doi:10.1158/1078-0432.CCR-07-4453. PMID 18316551.
- Vaidya, Jayant S; Joseph, David J; Tobias, Jeffrey S; et al. (July 2010). "Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial". The Lancet. 376 (9735): 91–102. doi:10.1016/S0140-6736(10)60837-9. PMID 20570343.
- Hill, M. A. (15 December 2004). "The variation in biological effectiveness of X-rays and gamma rays with energy". Radiation Protection Dosimetry. 112 (4): 471–481. doi:10.1093/rpd/nch091. PMID 15623881.
- Vaidya JS, Baum M, Tobias JS, Morgan S, D'Souza D (June 2002). "The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer". Eur J Surg Oncol. 28 (4): 447–54. doi:10.1053/ejso.2002.1275. PMID 12099658.
- Vaidya, Jayant S (31 October 2013). "IORT for breast cancer – the surgical technique of TARGIT". Retrieved 26 July 2016.
- "International Society of Intraoperative Radiation Therapy". Retrieved 26 July 2016.