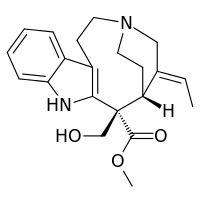
Stemmadenine
Stemmadenine is a terpene indole alkaloid. Stemmadenine is believed to be formed from preakuammicine by a carbon-carbon bond cleavage. Cleavage of a second carbon-carbon bond is thought to form dehydrosecodine.[1] The enzymes forming stemmadenine and using it as a substrate remain unknown to date.
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (1R,2S,16E)-16-ethylidene-2-(hydroxymethyl)-4,14-diazatetracyclo[12.2.2.03,11.05,10]octadeca-3(11),5,7,9-tetraene-2-carboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H26N2O3 | |
| Molar mass | 354.450 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Scott, Alastair I; Qureshi, Asaf A (1969). "Biogenesis of Strychnos, Aspidosperma, and Iboga alkaloids. Structure and reactions of preakuammicine". Journal of the American Chemical Society. 91 (21): 5874. doi:10.1021/ja01049a032. PMID 5812148.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.