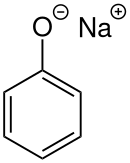
Sodium phenoxide
Sodium phenoxide (sodium phenolate) is an organic compound with the formula NaOC6H5. It is a white crystalline solid. Its anion, phenoxide, also known as phenolate, is the conjugate base of phenol. It is used as a precursor to many other organic compounds, such as aryl ethers.
 | |
| Names | |
|---|---|
| Other names
Sodium phenolate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.862 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5NaO | |
| Molar mass | 116.09 g/mol |
| Appearance | White solid |
| Hazards | |
| Main hazards | Harmful, Corrosive |
| Flash point | Nonflammable |
| Nonflammable | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and structure
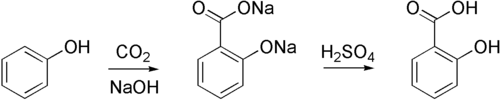
Most commonly, solutions of sodium phenoxide are produced by treating phenol with sodium hydroxide.[1] Anhydrous derivatives can be prepared by combining phenol and sodium:
- Na + HOC6H5 → NaOC6H5 + 1/2 H2
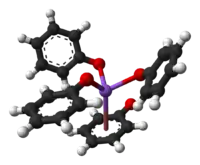
Like other sodium alkoxides, crystalline sodium phenolate adopts a complex structure involving multiple Na-O bonds. Solvent-free material is polymeric, each Na center being bound to three oxygen ligands as well as the phenyl ring. Adducts of sodium phenoxide are molecular, such as the cubane-type cluster [NaOPh]4(HMPA)4.[2]
Sodium phenoxide can be produced by the "alkaline fusion" of benzenesulfonic acid, whereby the sulfonate groups are displaced by hydroxide:
- C6H5SO3Na + 2 NaOH → C6H5ONa + Na2SO3
This route once was the principal industrial route to phenol.
Reactions
Sodium phenoxide is a moderately strong base. Acidification gives phenol:[3]
- PhOH ⇌ PhO− + H+ (K = 10−10)
Alkylation affords phenyl ethers:[1]
- NaOC6H5 + RBr → ROC6H5 + NaBr
The conversion is an extension of the Williamson ether synthesis. With acylating agents, one obtains esters:
- NaOC6H5 + RC(O)Cl → RCO2C6H5 + NaCl
Sodium phenoxide is susceptible to certain types of electrophilic aromatic substitutions. For example, it reacts with carbon dioxide to form 2-hydroxybenzoate, the conjugate base of salicylic acid. In general however, electrophiles irreversibly attack the oxygen center in phenoxide.
Uses
Sodium Phenoxide destroys or inhibits the growth of microorganisms, so people use it to prevent odor and cleanse skin. Sodium Phenoxide prevents or slows down the growth of bacteria, so it can protect cosmetics and personal care products.[4]
References
- C. S. Marvel, A. L. Tanenbaum (1929). "γ-Phenoxypropyl Bromide". Org. Synth. 9: 72. doi:10.15227/orgsyn.009.0072.CS1 maint: uses authors parameter (link)
- Michael Kunert, Eckhard Dinjus, Maria Nauck, Joachim Sieler "Structure and Reactivity of Sodium Phenoxide - Following the Course of the Kolbe-Schmitt Reaction" Chemische Berichte 1997 Volume 130, Issue 10, pages 1461–1465. doi:10.1002/cber.19971301017
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- "Sodium Phenoxide | Cosmetics Info". cosmeticsinfo.org. Retrieved 2020-06-19.
External links
![]() Media related to Sodium phenoxide at Wikimedia Commons
Media related to Sodium phenoxide at Wikimedia Commons