Sharp waves and ripples
Sharp waves and ripples (SWRs) are oscillatory patterns in the mammalian brain hippocampus seen on an EEG during immobility and sleep. There are three major network oscillation patterns in the hippocampus: theta waves, SWRs and gamma waves. Gamma oscillations are found in all major brain structures, whereas theta and sharp waves are specific to the hippocampus and its neighbouring areas. SWRs are composed of large amplitude sharp waves in local field potential and associated fast field oscillations known as ripples. SWRs are shown to be involved in memory consolidation and the replay of wakefulness-acquired memory. These network oscillations are the most synchronous patterns in the brain, making them susceptible to pathological patterns such as epilepsy.
History and background
Neuronal oscillations are important components of neuroscience research. During the last two decades, hippocampal oscillations have been a major focus in the research of neuronal oscillations.[1] Among different oscillations present in the brain, SWRs are the first and only population activity that start in the developing hippocampus, but they are the least understood network pattern of the hippocampus.[2]
Originally, these large waves were observed by Cornelius Vanderwolf in 1969, and later John O'Keefe investigated SPW-Rs in more detail in 1978 while studying the spatial memory of rats.[1] György Buzsáki and his collaborators studied and characterized SWRs in detail and described their physiological functions and role in different states of the animal.[1][3]
These patterns are large amplitude, aperiodic recurrent oscillations occurring in the apical dendritic layer of the CA1 regions of the hippocampus. Sharp waves are followed by synchronous fast field oscillations (140–200 Hz frequency), named ripples.[4]
Features of these oscillations provided evidences for their role in inducing synaptic plasticity and memory consolidation. Among these features are their widespread effect on the population neurons in the hippocampus, and the experience-dependent content of participating neurons. Studies have shown that elimination of SWRs by electrical stimulation interfered with rats ability to recall the spatial memory information.[5][6] These features support functional role of sharp waves and ripples in memory consolidation.
Hippocampal formation
Circuit
.png.webp)
The trisynaptic loop, as the main circuit of the hippocampus responsible for information transfer between the hippocampus and the cortex, is also the circuit producing SWRs. This circuit provides the pathway by which SWRs affect the cortical areas, and also receive inputs from them. Consequently, this loop is shown to be the pathway responsible for conversion of short term memory to long term memory. The trisynaptic loop of the hippocampus is one of the most thoroughly studied circuits for long-term potentiation.
Participant neuronal populations
Emergence of these self-organized hippocampal events are dependent on interactions between various pyramidal and granule neurons with different types of the interneurons in this circuit. Pyramidal cells of CA3 and CA1 dendritic layer region are important in generating these waves, and they affect the subiculum, parasubiculum, entorhinal cortex, and ultimately neurons of the neocortex.[2] During SWRs, which last approximately 100 milliseconds, 50,000-100,000 neurons discharge in synchrony, making SWRs the most synchronous event in the brain.[2] An important concept about the neuronal populations participating in these events is the fact that they are experience-dependent. Sequences that have been active during the animal's activity are the ones participating in SWRs. Activity naturally spreads along the pathways that have stronger synapses. This is one of the features of SWRs providing evidence for their role in memory consolidation.
Self-emergent network
Population bursts of pyramidal cells in the CA3 region of the hippocampus via CA3 collaterals cause depolarization of pyramidal cells in the dendritic layer of the CA1 which give rise to extracellular negative waves–the sharp waves–followed by fast ripples.[7] Discharge of pyramidal cells of CA3 region also activates the GABAergic interneurons.[2] Sparse firing of CA1 pyramidal cells and in-phase inhibition from the activated interneurons, give rise to high frequency (200 Hz) network oscillations, which are the ripples.[8] CA1 population bursts lead to highly synchronized activity in the target population of parahippocampal structures.[9]
Effects of neocortical inputs
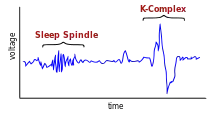
In spite of the self-emergent nature of the SWRs, their activity could be altered by inputs from the neocortex via the trisynaptic loop to the hippocampus. Activity of the neocortex during slow wave sleep is deterministic of inputs to the hippocampus; thalamocortical sleep spindles and delta waves are the sleep patterns of the neocortex.[10] These inputs contribute to the selection of different neuronal assemblies for initiation of SWRs, and affect the timing of the SWRs.[2] Different thalamocortical neuronal assemblies give rise to sleep spindles, and these cell assemblies affect the burst initiator for the sharp waves. In this manner, thalamocortical inputs affect the content of the SWRs going to neocortex.
Memory consolidation
Sharp waves and associated ripples are present in the mammalian brains of the species that have been investigated for this purpose, including mice, rats, rabbits, monkeys and humans.[4] In all of these species, they have been shown primarily to be involved in consolidation of recently acquired memories during the immobility and slow wave sleep. Characteristics of these oscillations, such as having experience dependent neuronal content, being affected by the cortical input, and reactivating neocortical pathways formed through recent experiences, provides evidences for their role in memory consolidation. Besides, some direct evidences for their role come from studies, investigating effects of their removal. Animal studies indicated that depletion of ripple activity by electrical stimulation, would impair formation of new memories in rats.[6][5] Furthermore, in spatially non-demanding tasks, such as passive exploration, optogenetic disruption of SPW-R events interferes with the stabilisation of the newly formed hippocampal place cell code (ref,[11] but see ref[12]). As for humans, what is currently known is that the hippocampus as a whole is important for memory consolidation. The most famous human patient with hippocampal lesion is H.M., whose hippocampus has been removed from both sides of his brain. Studies conducted on HM have clearly proven the role of the hippocampus in shaping the long-term memory.[13] Investigations also provide strong evidence supporting role of the hippocampus in consolidation of specific types of memories such as declarative and spatial memories.[1] However, clear evidence for the role of SPW-R events in memory consolidation in the hippocampus of humans is still missing.
Two stage model of memory
Based on the research findings about SPW-Rs, in 1989 an influential two stage model of memory proposed by Buzsáki, that subsequent evidences supported it. Based on this model initial memories of the events are formed during the acquisition and reinforced during replay. Acquisition occurs by theta and gamma waves activating a neuronal pathway for initial formation of the memory. Later this pathway would get replayed following the SPW-Rs propagation to neocortex. Neuronal sequences during replay happen in a faster rate and are in both forward and reverse direction of the initial formation.[3]
Ripples and fast gamma
In spite of the fact that hippocampal ripples (140–220 Hz) and fast gamma (90–150 Hz) oscillations have similar mechanisms of generation, they are two distinct patterns in the hippocampus. They are both produced as the response of the CA1 region to inputs from the CA3 region. Ripples are only present when theta waves are relatively absent during sharp waves, whereas fast gamma waves occur during theta waves and sharp waves.[9] The magnitude and frequency of both ripples and fast gamma patterns are dependent on the magnitude of hippocampal sharp waves. Stronger excitation from sharp waves results in ripple oscillations, whereas weaker stimulations generate fast gamma patterns.[14] Besides they are shown to be region dependent, ripples that are the fastest oscillations are present in the CA1 region pyramidal cells while gamma oscillations dominate in CA3 region and parahippocampal structures.[9]
Disease state
Epilepsy
In addition to ongoing research on the role of SPW-R complexes in memory consolidation and neuronal plasticity, another major area of the attention is their role in development of epilepsy. As mentioned before, SPW-Rs are the most synchronous oscillations observed in the brain; which implies any abnormal activity in this network would bring significant consequences. One of the deviations from normal activity is fast ripples. Fast ripples are a pathologic pattern that emerges from the physiologic ripples. These fast ripples are field potentials of hyper-synchronous bursting of excitatory neurons pyramidal cells at frequencies between 250–600 Hz.[15] Fast ripples activities in the hippocampus considered as pathologic patterns directly associated with epilepsy, but they appear as both physiologic and pathologic activity in neocortex.[16] Although underlying physiology and identifying contributions of fast ripples in generation of seizures are still under investigation and further research, studies are suggesting that fast ripples could be used as a biomarker of epileptogenic tissues.[17]
See also
Other brain waves
- Delta wave – (0.1–4 Hz)
- Alpha wave – (8–12 Hz)
- Theta wave – (4–8 Hz)
- Mu wave – (8–13 Hz)
- Beta wave – (13–30 Hz)
- Gamma wave – (25–100 Hz)
References
- Maier, N; Draguhn A.; Schmitz D; Both M. (March 2013). "Fast network oscillations in the hippocampus Phenomena, mechanisms and open questions at the interface of cellular and systemic neurosciences". e-Neuroforum. 4 (1): 1–10. doi:10.1007/s13295-013-0038-0.
- Buzsáki, György (2006). Rhythms of the brain. New York: Oxford Univ. Press. pp. 344–349. ISBN 978-0-19-982823-4.
- Buzsáki, Györgyi (1989). "Two-stage model of memory trace formation: a role for "noisy" brain states". Neuroscience. 31 (3): 551–570. doi:10.1016/0306-4522(89)90423-5. PMID 2687720. S2CID 23957660.
- Logothetis, N. K.; O. Eschenko; Y. Murayama; M. Augath; T. Steudel; H. C. Evard; M. Besserve; A. Oeltermann (November 2012). "Hippocampal–cortical interaction during periods of subcortical silence". Nature. 491 (7425): 547–553. Bibcode:2012Natur.491..547L. doi:10.1038/nature11618. PMID 23172213. S2CID 4416746.
- Girardeau, Gabrielle; Karim Benchenane; Sidney I Wiener; György Buzsáki; Michaël B Zugaro (September 2009). "Selective suppression of hippocampal ripples impairs spatial memory". Nature Neuroscience. 12 (10): 1222–1223. doi:10.1038/nn.2384. PMID 19749750. S2CID 23637142.
- Ego-Stengel, Valérie; Matthew A. Wilson (January 2010). "Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat" (PDF). Hippocampus. 20 (1): 1–10. doi:10.1002/hipo.20707. PMC 2801761. PMID 19816984.
- Buzsaki, Gyorgy; Horvath Z; Urioste R; Hetke J; Wise K (15 May 1992). "High-frequency network oscillation in the hippocampus". Science. 256 (5059): 1025–1027. Bibcode:1992Sci...256.1025B. doi:10.1126/science.1589772. PMID 1589772.
- Ylinen, Aaren; Anatol Bragin; Zoltan Nadasdy; Gabor Jando; Imre Sezabo; Attila Sik; G. Buzsáki (January 1995). "Sharp Wave-Associated High-Frequency Oscillation (200 Hz) in the Intact Hippocampus: Network and Intracellular Mechanism". The Journal of Neuroscience. 15 (1 Pt 1): 30–46. doi:10.1523/JNEUROSCI.15-01-00030.1995. PMC 6578299. PMID 7823136.
- Buzsáki, György; Fernando Lopes da Silvab (March 2012). "High frequency oscillations in the intact brain". Progress in Neurobiology. 98 (3): 241–249. doi:10.1016/j.pneurobio.2012.02.004. PMC 4895831. PMID 22449727.
- Sirota, Anton; Jozsef Csicsvari; Derek Buhl; Györgyi Buzsáki (February 2003). "Communication between neocortex and hippocampus during sleep in rodents". Proceedings of the National Academy of Sciences of the United States of America. 100 (4): 2065–2069. Bibcode:2003PNAS..100.2065S. doi:10.1073/pnas.0437938100. PMC 149959. PMID 12576550.
- van de Ven GM, Trouche S, McNamara CG, Allen K, Dupret D (7 Dec 2016). "Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples". Neuron. 92 (5): 968–974. doi:10.1016/j.neuron.2016.10.020. PMC 5158132. PMID 27840002.
- Kovacs KA, O'Neill J, Schoenenberger P, Penttonen M, Ranguel Guerrero DK, Csicsvari J (19 Nov 2016). "Optogenetically Blocking Sharp Wave Ripple Events in Sleep Does Not Interfere with the Formation of Stable Spatial Representation in the CA1 Area of the Hippocampus". PLOS ONE. 11 (10): e0164675. Bibcode:2016PLoSO..1164675K. doi:10.1371/journal.pone.0164675. PMC 5070819. PMID 27760158.
- Scoville, William Beecher; Brenda Milner (February 1957). "Loss of recent memory after bilateral hippocampal lesion". Neurology Neurosurgery Psychiatry. 20 (1): 11–21. doi:10.1136/jnnp.20.1.11. PMC 497229. PMID 13406589.
- Sullivan, David; Jozsef Csicsvari; Kenji Mizuseki; Sean Montgomery; Kamran Diba; György Buzsáki (June 2011). "Relationships between hippocampal sharp waves, ripples and fast gamma oscillation: influence of dentate and entorhinal cortical activity". Neuroscience. 31 (23): 8605–8616. doi:10.1523/JNEUROSCI.0294-11.2011. PMC 3134187. PMID 21653864.
- Bragin A, Engel J Jr, Wilson CL, Fried I, Mathern GW (February 1999). "Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures". Epilepsia. 40 (2): 127–37. doi:10.1111/j.1528-1157.1999.tb02065.x. PMID 9952257. S2CID 45089490.
- Köhling, Rüdiger; Staley Kevin (April 2011). "Network mechanisms for fast ripple activity in epileptic tissue". Epilepsy Research. 97 (3): 318–323. doi:10.1016/j.eplepsyres.2011.03.006. PMC 3152631. PMID 21470826.
- Jacobs, J.; P. Levan; C.E. Chatillon; A. Olivier; F. Dubeau; J. Gotman (March 2009). "High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type". Brain. 132 (4): 1022–1037. doi:10.1093/brain/awn351. PMC 3792079. PMID 19297507.
Further reading
- Buzsáki, György (2006). Rhythms of the brain. New York: Oxford Univ. Press. pp. 344–349. ISBN 978-0-19-982823-4.