Saccharolipid
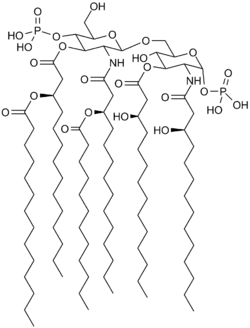
Saccharolipids are chemical compounds belonging to the general category of glycolipids. Their chemical structure involves fatty acids linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a monosaccharide substitutes for the glycerol backbone present in glycerolipids and glycerophospholipids. The most familiar saccharolipids are the acylated glucosamine precursors of the lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in Escherichia coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine (LipidA) that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.[2]
See also
References
- Raetz, Christian R. H.; Guan, Ziqiang; Ingram, Brian O.; Six, David A.; Song, Feng; Wang, Xiaoyuan; Zhao, Jinshi (2009). "Discovery of new biosynthetic pathways: the lipid A story". Journal of Lipid Research. 50 Suppl: S103–S108. doi:10.1194/jlr.R800060-JLR200. PMC 2674688. PMID 18974037.
- Raetz CR; Garrett TA; Reynolds CM; Shaw WA; Moore JD; Smith DC Jr; Ribeiro AA; Murphy RC; Ulevitch RJ; Fearns C; Reichart D; Glass CK; Benner C; Subramaniam S; Harkewicz R; Bowers-Gentry RC; Buczynski MW; Cooper JA; Deems RA; Dennis EA (2006). "Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4". Journal of Lipid Research. 47 (5): 1097–1111. doi:10.1194/jlr.M600027-JLR200. PMID 16479018.