Pulsed electromagnetic field therapy
Pulsed electromagnetic field therapy (PEMFT, or PEMF therapy), also known as low field magnetic stimulation (LFMS) uses electromagnetic fields in an attempt to heal non-union fractures and depression.[1] By 2007 the FDA had cleared several such stimulation devices.[2]
| Pulsed electromagnetic field therapy | |
|---|---|
 Drolet's 1990 Rhumart system, a PEMF device. | |
| Other names | pulsed magnetic therapy, pulse magnetotherapy (PEMF) |
In 2013 the U.S. Food and Drug Administration (FDA) warned a manufacturer for promoting the device for unapproved uses such as cerebral palsy and spinal cord injury.[3]
Use
Delayed- and non-union fractures
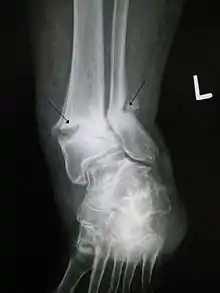
In the United States, PEMF exposure is an FDA-approved treatment of fracture nonunions. There is considerable clinical evidence to support the use of PEMF for fracture nonunions with reported healing rates for nonunions after PEMF stimulation between 73% and 85%.[4][5] While PEMF therapy is claimed to offer some benefits in the treatment of fractures, the evidence is inconclusive and is insufficient to inform current clinical practice.[6] PEMFs is generally not among the guidelines to treat bone and osteochondral defects. Notwithstanding, there is strong evidence for ELF-PEMF treatment.[7] Pulsed Electromagnetic Fields promote the synthesis of skeletal extracellular matrix. The physiologic process of the response of skeletal cells to PEMF is the synthesis of extracellular matrix structural and signaling molecules in the wound. The result of the signaling processes is to instruct skeletal cells to synthesize structural extracellular matrix and signaling molecules and enhance the ability of skeletal tissues to respond to changing physicochemical environments and biomechanical demands, and facilitate healing.[8] A reduction in time to union and an increase in the percentage of fracture healing in PEMF-stimulated patients compared with controls was reported in fresh tibial fractures,[9] and in femoral neck fractures.[10]
History
Prior to the year 2000, in parallel with the PEMF research being done in Western Europe, the United States, and Japan, a great deal of scientific work was being done in scientific isolation behind the Iron Curtain, as summarized in a detailed technical report,[11] showing scientific evidence for promising benefits from the use of PEMF for a very wide range of applications including peripheral vascular disease, lung disease, gastrointestinal disease, neurological disease, rheumatic disease, pediatrics, dermatology, surgery, gynecology, oral medicine, otorhinolaryngology, ophthalmology, immunity, inflammation, reproduction, and tumors, based on over 200 referenced scientific papers involving both human and animal studies.[11]
Veterinarians were the first health professionals to use PEMF therapy, usually to attempt to heal broken legs in racehorses.[12] In 2004, a pulsed electromagnetic field system was approved by the FDA as an adjunct to cervical fusion surgery in patients at high risk for non-fusion.[12] On 8/9 September 2020 the FDA recommended to shift PEMF wellness devices from the Class 3 category to a Class 2 status.{[13]} PEMF devices that have been FDA cleared are able to make health claims that require a doctor's prescription for use.[14]
Although claims that electricity might aid bone healing was reported as early as 1841, it was not until the mid-1950s that scientists seriously studied the subject. During the 1970s, Bassett and his team introduced a new approach which attempted to treat delayed fractures; a technique that employed a very specific biphasic low frequency signal to be applied for non-union/delayed fractures.[15][16][17][18] The use of electrical stimulation in the lumbosacral region was first attempted by Alan Dwyer of Australia.[19][20]
Wellness devices
The original PEMF devices consisted of a Helmholtz coil which generated a magnetic field. The patient's body was placed inside the magnetic field to deliver treatment. Today, the majority of PEMF wellness devices resemble a typical yoga mat in dimensions but are slightly thicker to house several flat spiral coils to produce an even electromagnetic field. A frequency generator is then used to energize the coils to create a pulsed electromagnetic field. A wide variety of professional and consumer PEMF devices are sold and marketed as FDA registered wellness devices.[14] The majority are manufactured in Germany, Austria and Switzerland and are imported into North America as electric massagers or full body electric yoga mats. They are either placed on a massage table for clinical use or directly on the floor in the home to practice simple yoga postures. The companies that sell and manufacture them as "general wellness products" are not permitted to make medical claims of effectiveness in treating disease.[14]
Research
Knee osteoarthritis
A 2013 review found that evidence was of very low quality, there might be a benefit for improved function, and there was no evidence for benefit for pain.[21]
In 2017 the wearable ActiPatch PEMF Device was FDA 510k Cleared, Application # K152432, for "Adjunctive treatment of musculoskeletal pain related to: (1) plantar fasciitis of the heel; and (2) osteoarthritis of the knee". This Clearance was for Over the Counter use.[22]
Depression
Use of pulsed electromagnetic field therapy has been studied for depression, where it was postulated that "The antidepressant effect of tPEMF may be specifically attributable to its effects on local brain activity and connectivity."[23]
Postoperative Pain
In 2019 the wearable RecoveryRx PEMF Device was FDA Cleared, Application K190251, for "Adjunctive treatment of postoperative pain".[24]
Musculoskeletal Pain
In January 2020 the wearable ActiPatch was granted FDA 510k Clearance, Application # K192234, for Over the Counter Marketing relating to "Adjunctive treatment of musculoskeletal pain",.[25] The manufacturer of ActiPatch, Bioelectronics Corp, submitted 3 Clinical Studies to support the efficacy of ActiPatch relating to musculoskeletal pain,.[25]
References
- Martiny, K; Lunde, M; Bech, P (15 July 2010). "Transcranial low voltage pulsed electromagnetic fields in patients with treatment-resistant depression". Biological Psychiatry. 68 (2): 163–9. doi:10.1016/j.biopsych.2010.02.017. PMID 20385376.
- Markov, Marko S (2007). "Expanding Use of Pulsed Electromagnetic Field Therapies". Electromagnetic Biology & Medicine. 26 (3): 257–274. doi:10.1080/15368370701580806. PMID 17886012.
- "Warning Letters - Curatronic Ltd. 1/9/13". www.fda.gov. Archived from the original on 26 July 2018. Retrieved 7 January 2018.
- Assiotis, Aggelos; Sachinis, Nick P.; Chalidis, Byron E. (2012-06-08). "Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature". Journal of Orthopaedic Surgery and Research. 7 (1): 24. doi:10.1186/1749-799X-7-24. ISSN 1749-799X. PMC 3441225. PMID 22681718.
- Cadossi, Ruggero; Massari, Leo; Racine-Avila, Jennifer; Aaron, Roy K. (2020-05-18). "Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response". JAAOS Global Research & Reviews. 4 (5). doi:10.5435/JAAOSGlobal-D-19-00155. ISSN 2474-7661. PMC 7434032.
- Griffin, XL; Costa, ML; Parsons, N; Smith, N (13 April 2011). "Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults". The Cochrane Database of Systematic Reviews (4): CD008471. doi:10.1002/14651858.CD008471.pub2. PMID 21491410.
- Ehnert, Sabrina; Schröter, Steffen; Aspera-Werz, Romina H.; Eisler, Wiebke; Falldorf, Karsten; Ronniger, Michael; Nussler, Andreas K. (December 2019). "Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery". Journal of Clinical Medicine. 8 (12): 2028. doi:10.3390/jcm8122028.
- Cadossi, Ruggero; Massari, Leo; Racine-Avila, Jennifer; Aaron, Roy K. (May 2020). "Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response". JAAOS Global Research & Reviews. 4 (5): e19.00155. doi:10.5435/JAAOSGlobal-D-19-00155. ISSN 2474-7661.
- Fontanesi, G.; Traina, G. C.; Giancecchi, F.; Tartaglia, I.; Rotini, R.; Virgili, B.; Cadossi, R.; Ceccherelli, G.; Marino, A. A. (September 1986). "Slow healing fractures: can they be prevented? (Results of electrical stimulation in fibular osteotomies in rats and in diaphyseal fractures of the tibia in humans)". Italian Journal of Orthopaedics and Traumatology. 12 (3): 371–385. ISSN 0390-5489. PMID 3494709.
- Faldini, Cesare; Cadossi, Matteo; Luciani, Deianira; Betti, Emanuele; Chiarello, Eugenio; Giannini, Sandro (May 2010). "Electromagnetic bone growth stimulation in patients with femoral neck fractures treated with screws: Prospective randomized double-blind study". Current Orthopaedic Practice. 21 (3): 282–287. doi:10.1097/BCO.0b013e3181d4880f. ISSN 1940-7041.
- Jerabeck, J; Pawluk, W (1998). Magnetic therapy in eastern Europe : a review of 30 years of research. W. Pawluk. ISBN 0-9664227-0-8.
- "Electrical stimulation of the spine as an adjunct to spinal fusion procedures". Blue Cross & Blue Shield of Mississippi. Archived from the original on 2015-04-02.
Pulsed electromagnetic field systems with FDA PMA include the EBI Bone Healing System from Electrobiology, Inc., which was first approved in 1979 and indicated for nonunions, failed fusions, and congenital pseudarthroses; and the Cervical-Stim from Orthofix, which was approved in 2004 as an adjunct to cervical fusion surgery in patients at high risk for non-fusion.
- https://www.fda.gov/media/141850/download
- "General Wellness: Policy for Low Risk Devices - Guidance for Industry and Food and Drug Administration Staff" (PDF). U.S. Food and Drug Administration. 29 July 2016. Retrieved 16 February 2016.
- Bassett CA, Pawluk RJ, Pilla AA; Pawluk; Pilla (1974). "Acceleration of fracture repair by electromagnetic fields. A surgically noninvasive method". Ann N Y Acad Sci. 238 (1): 242–62. Bibcode:1974NYASA.238..242B. doi:10.1111/j.1749-6632.1974.tb26794.x. PMID 4548330.CS1 maint: multiple names: authors list (link)
- Bassett CA, Pawluk RJ, Pilla AA; Pawluk; Pilla (1974). "Augmentation of Bone Repair by Inductively Coupled Electromagnetic Fields". Science. 184 (4136): 575–7. Bibcode:1974Sci...184..575B. doi:10.1126/science.184.4136.575. PMID 4821958.CS1 maint: multiple names: authors list (link)
- Bassett CA, Pilla AA, Pawluk RJ; Pilla; Pawluk (1977). "A non-operative salvage of surgically-resistant pseudarthroses and non-unions by pulsing electromagnetic fields. A preliminary report". Clin Orthop. 124 (124): 128–43. doi:10.1097/00003086-197705000-00017. PMID 598067.CS1 maint: multiple names: authors list (link)
- Bassett CA, Mitchell SN, Norton L, Pilla A; Mitchell; Norton; Pilla (1978). "Repair of non-unions by pulsing electromagnetic fields". Acta Orthop Belg. 44 (5): 706–24. PMID 380258.CS1 maint: multiple names: authors list (link)
- Mackenzie, Donald, Francis D Veninga; Veninga (2004). "Reversal of delayed union of anterior cervical fusion treated with pulsed electromagnetic field stimulation: case report". Southern Medical Journal. 97 (5): 519–524. doi:10.1097/00007611-200405000-00021. PMID 15180031.CS1 maint: multiple names: authors list (link)
- Bose, B (2001). "Outcomes after posterolateral lumbar fusion with instrumentation in patients treated with adjunctive pulsed electromagnetic field stimulation". Advances in Therapy. 18 (1): 12–20. doi:10.1007/BF02850247. PMID 11512529.
- Negm, A; Lorbergs, A; Macintyre, NJ (September 2013). "Efficacy of low frequency pulsed subsensory threshold electrical stimulation vs placebo on pain and physical function in people with knee osteoarthritis: systematic review with meta-analysis". Osteoarthritis and Cartilage. 21 (9): 1281–9. doi:10.1016/j.joca.2013.06.015. PMID 23973142.
- https://www.accessdata.fda.gov/cdrh_docs/pdf15/K152432.pdf
- van Belkum, SM; Bosker, FJ; Kortekaas, R; Beersma, DG; Schoevers, RA (3 November 2016). "Treatment of depression with low-strength transcranial pulsed electromagnetic fields: A mechanistic point of view". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 71: 137–43. doi:10.1016/j.pnpbp.2016.07.006. PMID 27449361.
- https://www.accessdata.fda.gov/cdrh_docs/pdf19/K190251.pdf
- https://www.accessdata.fda.gov/cdrh_docs/pdf19/K192234.pdf