Potassium ethoxide
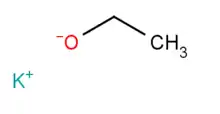
Potassium ethoxide, also known as potassium ethanolate, is an off-white or yellow powder with the chemical formula of C2H5KO. Potassium ethoxide contains an ethoxide ion, the conjugate base of ethanol, which makes this compounds strongly basic. It hydrolyzes to yield ethanol and potassium hydroxide.
 | |
| Names | |
|---|---|
| IUPAC name
Potassium ethanolate | |
| Other names
Potassium ethylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.011.845 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5KO | |
| Molar mass | 84.159 g·mol−1 |
| Appearance | Yellow or Off-White Powder[1] |
| Density | 0.894 g/mL [2] |
| Melting point | 250 °C (482 °F; 523 K) |
| Reacts | |
| Hazards | |
EU classification (DSD) (outdated) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Potassium ethoxide is used as a strong base, similar to sodium and potassium methoxides, and potassium tert-butoxide. Catalytic amounts of potassium ethoxide in ethanol can be used to perform transesterification reactions that yield ethyl esters. Sodium or potassium ethoxide is also a suitable base for the malonic ester synthesis where diethyl malonate is used, since any transesterification reaction does not result in ester scrambling.
Safety
Potassium ethoxide is stable, but also both flammable and corrosive. The compound reacts vigorously with water. If the compound comes into contact with damp air, it may lead to the heating and ignition of the solid powder. Keep separated from air, moisture, water, acids, oxidizing agents, and reducing agents.[3] It can also cause severe skin burns.[4]
References
- , n.pag. World Of Chemicals. Web. 19 Oct 2012. <http://www.worldofchemicals.com/wochem/pub/chempotassium-ethoxide.html>.
- CID 23670592 from PubChem
- , n.pag. CasLAb. Web. 19 Oct 2012. <http://www.caslab.com/Potassium_ethoxide_CAS_917-58-8/>.
- , n.pag. chemexper. Web. 19 Oct 2012. <http://www.chemexper.com/chemicals/supplier/cas/917-58-8.html>.