Pomarose
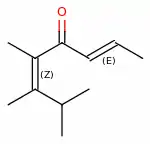
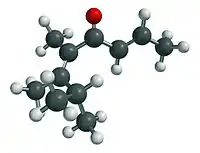
Pomarose is a high-impact captive odorant patented by Givaudan.[1] It is a double-unsaturated ketone that does not occur in nature. Pomarose has a powerful fruity rose odor with nuances of apples, plums and raisins, which is almost entirely due to the (2E,5Z)-stereoisomer, while its (2E,5E)-isomer is barely detectable for most people.[2] Catalyzed by traces of acids, both isomers equilibrate however quickly upon standing in glass containers.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5,6,7-Trimethylocta-2,5-dien-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H18O | |
| Molar mass | 166.264 g·mol−1 |
| Density | 0.855 g/cm3 |
| Boiling point | 236.2 °C (457.2 °F; 509.3 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Discovery and synthesis
5,6,7-Trimethylocta-2,5-dien-4-one was suspected by Philip Kraft et al.[3] by investigation of the NMR spectra of an unknown trace component with damascone odor in a crude complex reaction product. Although this trace component eventually turned out to be the constitutional isomer 2-methyl-3-isopropylhepta-2,5-dien-4-one. Pomarose was synthesized for structural curiosity and found to possess even superior fruity, rosy odor characteristics, reminiscent of apples, plums, raisins and other dried fruits with a low odor threshold of 0.5 ng/L air. The synthesis comprised borontrifluoride-catalyzed addition of methyl isopropyl ketone to 1-ethoxyprop-1-yne, which afforded ethyl 2,3,4-trimethylpent-2-enoate, and then was transformed into the target molecule by Grignard reaction with propen-1-ylmagnesium bromide via in situ enolization.
Use in perfumery
Pomarose has been used in a variety of perfumes.[4] It had its debut in Be Delicious for Men and it is also used in Unforgivable, 1 Million, CK free, Legend, Unforgivable Woman and John Galliano.
Related compounds
References
- Kraft, Philip (2001). "Preparation of 2-,5-,6-,7-,8-substituted oct-2-en-4-ones with odor and taste properties". Eur. Pat. Appl. 2001: EP 1149820 A1 20011031.
- Swift, Karl, ed. (2002). Advances in flavours and fragrances: From the sensation to the synthesis. Cambridge: Royal Society of Chemistry. p. 138. ISBN 9780854048212.
- Kraft, Philip; Denis, Caroline; Eichenberger, Walter (2001). "5,6,7-Trimethylocta-2,5-dien-4-one − A Suspected Odorant with Surprising Olfactory Properties". European Journal of Organic Chemistry. 2001 (12): 2363–2369. doi:10.1002/1099-0690(200106)2001:12<2363::AID-EJOC2363>3.0.CO;2-E.
- Ohloff, Günther; Pickenhagen, Wilhelm; Kraft, Philip. (2012). Scent and Chemistry – The Molecular World of Odors. Zurich: Verlag Helvetica Chimica Acta. p. 217. ISBN 9783906390666.