Polyisoprene
Polyisoprene is a collective name for polymers that are produced by polymerization of isoprene. cis-1,4-Polyisoprene, which is also called isoprene rubber, is a major ingredient of natural rubber. trans-1,4-Polyisoprene is a major ingredient of gutta-percha. Annual worldwide production of polyisoprene was 13 megatonnes in 2007.[1]

Properties
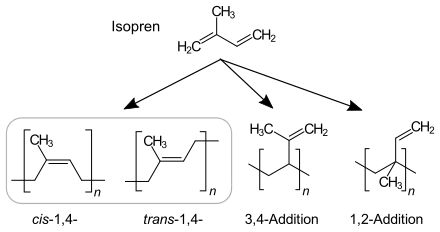
In principle, the polymerization of isoprene can result in four different isomers. The relative amount of each isomer in the polymer is dependent on the mechanism of the polymerization reaction.
Anionic chain polymerization, which is initiated by n-Butyllithium, produces cis-1,4-polyisoprene dominant polyisoprene. 90–92% of repeating units are cis-1,4-, 2–3% trans-1,4- and 6–7% 3,4-units.[2]
Coordinative chain polymerization: With Ziegler–Natta catalyst TiCl4/Al(i-C4H9)3, a more pure cis-1,4-polyisoprene similar to natural rubber is formed. With Ziegler–Natta catalyst VCl3/Al(i-C4H9)3, trans-dominant polyisoprene is formed.[3]
1,2 and 3,4 dominant polyisoprene is produced MoO2Cl2 catalyst supported by phosphorus ligand and Al(OPhCH3)(i-Bu)2 co-catalyst.[4]
Usage
Natural Gutta-percha and synthesized trans-1,4-polyisoprenes are used for golf balls. Natural rubber and synthesized cis-1,4-polyisoprene are used for elastomer.
Polyisoprene condoms provide an alternative to traditional latex condoms.
See also
References
- Sebastian Koltzenburg, Michael Maskos, Oskar Nuyken, Polymere: Synthese, Eigenschaften und Anwendungen, Springer, Berlin, 2012, S. 424.
- Jürgen Falbe, Manfred Regitz (Hrsg.): CD Römpp Chemie Lexikon, Thieme, Stuttgart, 1995.
- Bernd Tieke, Makromolekulare Chemie, 3. Auflage, Wiley-VCH, Weinheim, 2014, S. 149.
- 1,2- and 3,4-rich polyisoprene synthesized by Mo(VI)-based catalyst with phosphorus ligand Polymer Science Series B September 2016, Volume 58, Issue 5, pp 495–502