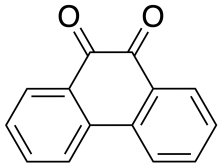
Phenanthrenequinone
Phenanthrenedione is a quinone derivative of a polycyclic aromatic hydrocarbon. It is an orange, water-insoluble solid.[2]
 | |
| Names | |
|---|---|
| Systematic IUPAC name
9,10-Phenanthrenequinone[1] | |
| Identifiers | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.377 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| C14H8O2 | |
| Molar mass | 208.216 g·mol−1 |
| Appearance | Orange solid |
| Odor | Odorless |
| Melting point | 209 °C (408 °F; 482 K) |
| Boiling point | 360 °C (680 °F; 633 K) |
| Slightly soluble (7.5 mg L−1) | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H315, H319, H400 | |
| P273, P264, P280, P305+351+338, P337+313, P302+352, P332+313, P362, P391, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Laboratory synthesis and use
It has been prepared by oxidation of phenanthrene with chromic acid.[3]
It is used as a artificial mediator for electron acceptor/donor in Mo/W containing formate dehydrogenase reduction of Carbon dioxide to formate and vice versa. It is a better electron acceptor than the natural Nicodinamide adenosine dinucleotide(NAD+).
Safety
It is a cytotoxic.[4]
References
- " 84-11-7|Phenanthrenequinone|Toxnet|". nih.gov.
- Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke (2000). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227.
- Wendland, Ray; LaLonde, John (1954). "Phenanthrenequinone". Org. Synth. 34: 76. doi:10.15227/orgsyn.034.0076.
- Robert A. Kanaly; Natsuko Hamamura (September 2013). "9,10-Phenanthrenedione biodegradation by a soil bacterium and identification of transformation products by LC/ESI-MS/MS". Chemosphere. 92 (11): 1442–1449. doi:10.1016/j.chemosphere.2013.03.054. PMID 23611246.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
