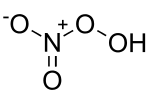
Peroxynitric acid
Peroxynitric acid or peroxonitric acid is a chemical compound with the formula HNO
4. It is an oxyacid of nitrogen, after peroxynitrous acid.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hydroxy nitrate | |||
| Systematic IUPAC name | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| HNO4 | |||
| Molar mass | 79.01224 g/mol | ||
| Conjugate base | Peroxynitrate | ||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Peroxynitrate, the conjugate base of peroxynitric acid, is formed rapidly during decomposition of peroxynitrite in neutral conditions.[4]
Atmospheric chemistry
Peroxynitric acid is formed in the atmosphere, although it is unstable, it is important as a reservoir for NO2 through the reversible radical reaction:[5]
- HO
2NO
2 ⇌ HO•
2 + NO•
2
References
- "Peroxynitric Acid - Compound Summary".
- "peroxynitric acid". PubChem. Retrieved 9 December 2012.
- "125239-87-4". ChemIndex. Retrieved 9 December 2012.
- Miyamoto, S; Ronsein, GE; Corrêa, TC; Martinez, GR; Medeiros, MH; Di Mascio, P (2009). "Direct evidence of singlet molecular oxygen generation from peroxynitrate, a decomposition product of peroxynitrite". Dalton Trans (29): 5720–9. doi:10.1039/b905560f. PMID 20449086.
- Finlayson-Pitts, Barbara J.; Pitts, James N. (2000), Chemistry of the Upper and Lower Atmosphere, Elsevier, p. 100, doi:10.1016/b978-012257060-5/50000-9, ISBN 978-0-12-257060-5, retrieved 2020-09-24
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.

