Pentafluorophenyl esters
Pentafluorophenyl (PFP) esters are chemical compounds with the generic formula RC(O)OC6F5. They are active esters derived from pentafluorophenol (HOC6F5).
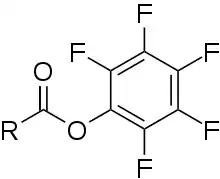
Generic structure of a pentafluorophenyl ester
PFP esters are useful for attaching fluorophores such as fluorescein[1] or haptens[2] to primary amines in biomolecules. They also are valuable in laboratory peptide synthesis. Pentafluorophenyl esters produce amide bonds as effectively as succinimidyl esters and various similar agents do, but PFP esters are particularly useful because they are less susceptible to spontaneous hydrolysis during conjugation reactions.[3]
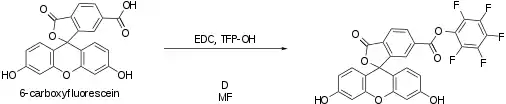
Scheme of pentafluorophenylester formation of 6-carboxyfluoroscein
References
- Hanai, T.; Hatano, H. (1996). Advances in Liquid Chromatography: 35 Years of Column Liquid Chromatography. World Scientific Publication Co. ISBN 978-981-02-1906-2.
- Deck, M. B.; Sjölin, P.; Unanue, E. R.; Kihlberg, J. (1999). "MHC-Restricted, Glycopeptide-Specific T Cells Show Specificity for Both Carbohydrate and Peptide Residues" (pdf). The Journal of Immunology. 162 (8): 4740–4744. PMID 10202015.
- Katz, J. (1998-12-15). "Advances in Peptide Coupling" (pdf). Harvard University.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.