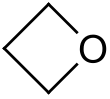
Oxetane
Oxetane, or 1,3-propylene oxide, is a heterocyclic organic compound with the molecular formula C
3H
6O, having a four-membered ring with three carbon atoms and one oxygen atom.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxetane[1] | |||
| Systematic IUPAC name
1,3-Epoxypropane Oxacyclobutane | |||
| Other names
1,3-Propylene oxide Trimethylene oxide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 102382 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.241 | ||
| EC Number |
| ||
| 239520 | |||
PubChem CID |
|||
| UNII | |||
| UN number | 1280 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.08 g/mol | ||
| Density | 0.8930 g/cm3 | ||
| Melting point | −97 °C (−143 °F; 176 K) | ||
| Boiling point | 49 to 50 °C (120 to 122 °F; 322 to 323 K) | ||
Refractive index (nD) |
1.3895 at 25°C | ||
| Hazards | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
| H225, H302, H312, H332 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+312, P302+352, P303+361+353, P304+312, P304+340, P312, P322, P330, P363, P370+378, P403+235, P501 | |||
| Flash point | −28.3 °C; −19.0 °F; 244.8 K (NTP, 1992) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The term "an oxetane" or "oxetanes" refer to any organic compound containing the oxetane ring.
Production
A typical well-known method of preparation is the reaction of potassium hydroxide with 3-chloropropyl acetate at 150 °C:[2]
Yield of oxetane made this way is c. 40%, as the synthesis can lead to a variety of by-products.
Anotherer possible reaction to form an oxetane ring is the Paternò–Büchi reaction. The oxetane ring can also be formed through diol cyclization as well as through decarboxylation of a six-membered cyclic carbonate.
Taxol
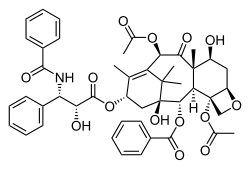
Paclitaxel (Taxol) is an example of a natural product containing an oxetane ring. Taxol has become a major point of interest among researchers due to its unusual structure and success in the involvement of cancer treatment.[3] The attached oxetane ring is an important feature that is used for the binding of microtubules in structure activity; however little is known about how the reaction is catalyzed in nature, which creates a challenge for scientists trying to synthesize the product.[3]
See also
- β-Propiolactone or 2-oxetanone.
- 3-Oxetanone
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- C. R. Noller (1955). "Trimethylene Oxide". Organic Syntheses. 29: 92.; Collective Volume, 3, p. 835
- Willenbring, Dan, and Dean J. Tantillo.. "Mechanistic possibilities for oxetane formation in the biosynthesis of Taxol’s D ring." Russian Journal of General Chemistry 78.4 (Apr. 2008): 7237–31. Advanced Placement Source. EBSCO. [Library name], [City], [State abbreviation]. 22 Apr. 2009 <http://search.ebscohost.com/login.aspx?direct=true&db=aqh&AN=32154883&site=ehost-live>