Oxepin
Oxepin is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. The parent C6H6O exists as an equilibrium mixture with benzene oxide. The oxepin–benzene oxide equilibrium is affected by the ring substituents.[1] A related dimethyl derivative exists mainly as the oxepin isomer, an orange liquid.[2]
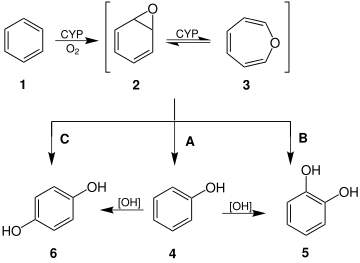
Formation and selected reactions of benzene oxide (2) and oxepin (3).
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Oxepine | |||
| Other names
Oxacycloheptatriene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H6O | |||
| Molar mass | 94.11 g/mol | ||
| Related compounds | |||
Related compounds |
Cyclohexene oxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Oxepin is an intermediate in the oxidation of benzene by the cytochrome P450 (CYP).[3] Other arene oxides are metabolites of the parent arene.
References
| Wikimedia Commons has media related to Oxepins. |
- E. Vogel, H. Günther (1967). "Benzene Oxide-Oxepin Valence Tautomerism". Angewandte Chemie International Edition in English. 6 (5): 385–401. doi:10.1002/anie.196703851.
- L. A. Paquette and J. H. Barrett (1969). "2,7-Dimethyloxepin". Org. Synth. 49: 62. doi:10.15227/orgsyn.049.0062.CS1 maint: uses authors parameter (link)
- R. Snyder, G. Witz, and B. D. Goldstein (1993). "The Toxicology of Benzene". Environmental Health Perspectives. 100: 293–306. doi:10.1289/ehp.93100293. JSTOR 3431535. PMC 1519582. PMID 8354177.CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.

